ProClin™ Preservatives
Used in over 1,000 FDA registered IVD kits from industry-leading diagnostics manufacturers, ProClin™ preservatives are water-soluble formulations that are among the most effective in the IVD industry. At low working concentrations, ProClin™ products help extend the shelf life of IVD reagents by effectively and immediately inhibiting a broad spectrum of microbes. ProClin™ preservatives are readily available in four unique formulations, ensuring a variety of options to meet a wide range of specific needs.
Products
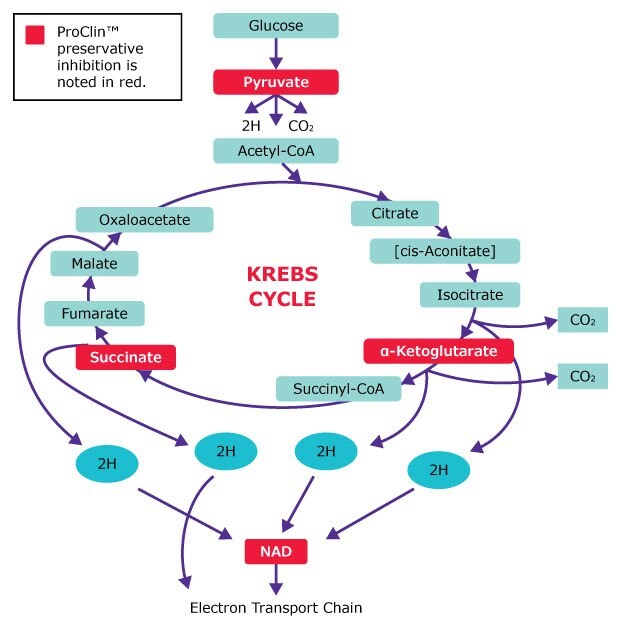
ProClin™ preservatives attack the Krebs cycle at four key points: the enzymes pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, succinate dehydrogenase, and NADH dehydrogenase. Because all bacteria and fungi possess at least part of the Krebs cycle, they are broad spectrum in their activity.
ProClin™ preservatives are readily available in convenient pack sizes suitable for all manufacturing processes and volumes. Along side our four formulations, we also provide ProClin™ support products like a ProClin™ reference standard and a ProClin™ variety kit, useful in testing and evaluating the use of ProClin™ preservative within a specific diagnostic reagent or assay.
The ProClin™ Variety Kit (48119-U) contains 5 mL bottles of all four formulations and is an excellent tool for evaluating the suitability of ProClin™ products for their specific applications. See the characteristic differentiators between the formulations in our technical chart. Want to see how ProClin™ preservatives benefit your reagent? Request a sample.
Despite the enhanced safety profile of ProClin™ products, a study from an independent lab has shown equivalent or improved efficacy when compared to top alternatives like thimerosal and sodium azide.
ProClin™ preservatives are restricted to use in IVD assay development and are intended for use in IVD manufacturing only.
Disclaimer
The product is not intended for use as a biocide under global biocide regulations, including but not limited to US EPA′s Federal Insecticide Fungicide and Rodenticide Act, European Biocidal Products Regulation, Canada’s Pest Management Regulatory Agency, Turkey’s Biocidal Products Regulation, Korea’s Consumer Chemical Products and Biocide Safety Management Act (K-BPR) and others.
We provide ProClin™ preservative product range with different characteristic formulations in multiple pack sizes to suit your specific needs.
Related Resources
- Brochure: ProClin™ Preservatives: Easy to incorporate into your diagnostic assay development
ProClin™ preservatives are used in over 1,000 FDA registered IVD kits from industry leading manufacturers. At low working concentrations, ProClin™ preservatives can help extend the shelf life of IVD reagents by effectively and immediately inhibiting a broad spectrum of microbes
- Flyer: For In Vitro Diagnostic Materials: Efficacy vs. Thimerosal and Sodium Azide
The traditional preservatives used to prevent microbe growth in in vitro diagnostic products are not ideal. Thimerosal is expensive and, because it contains mercury, is classified as toxic for disposal.
- FAQ: ProClin™ Preservatives Frequently Asked Questions
ProClinTM products are dedicated for use in the Medical Devices (MD) and in vitro diagnostics (IVD) sector globally. The use of biocides to treat MD and IVD products is exempted from the Biocidal Products Regulation (BPR) as long as the MD/IVD products are in scope of the MD/IVD directives/regulations.
- Technote: ProClin™ 300 Preservative
ProClin™ 300 preservative is a highly effective biocide for the control of microorganisms in reagents and products intended for in vitro diagnostic use. Due to its broadspectrum activity, low toxicity at recommended use levels, excellent compatibility, and stability, ProClin™ 300 biocide is the ideal choice as an effective preservative in diagnostic reagents.
To continue reading please sign in or create an account.
Don't Have An Account?