437727
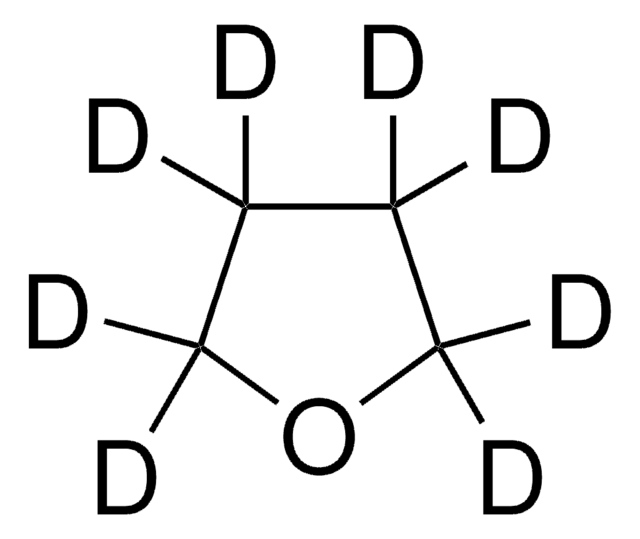
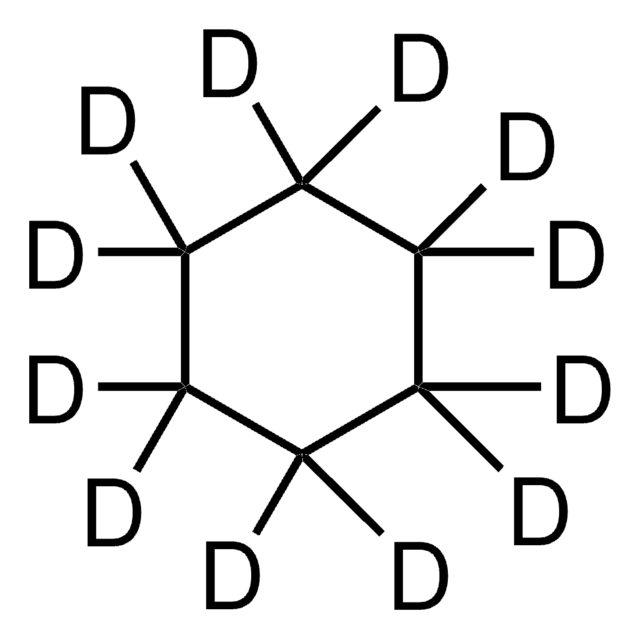
Tetrahydrofuran-d8
≥99.5 atom % D, contains 0.03 % (v/v) TMS
Synonym(s):
THF-d8, Deuterated tetrahydrofuran, Octadeuterotetrahydrofuran
About This Item
Recommended Products
isotopic purity
≥99.5 atom % D
Assay
≥99% (CP)
form
liquid
contains
0.03 % (v/v) TMS
technique(s)
NMR: suitable
impurities
≤0.03% water
water
refractive index
n20/D 1.403 (lit.)
bp
65-66 °C (lit.)
mp
−106 °C (lit.)
density
0.985 g/mL at 25 °C (lit.)
mass shift
M+8
storage temp.
2-8°C
SMILES string
[2H]C1([2H])OC([2H])([2H])C([2H])([2H])C1([2H])[2H]
InChI
1S/C4H8O/c1-2-4-5-3-1/h1-4H2/i1D2,2D2,3D2,4D2
InChI key
WYURNTSHIVDZCO-SVYQBANQSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Experimental and Theoretical Study of CO2 Insertion into Ruthenium Hydride Complexes.: This research provides insights into the mechanistic pathways for CO2 reactivity with ruthenium hydride complexes, potentially relevant for catalytic applications including transformations involving Tetrahydrofuran-d₈ (Ramakrishnan et al., 2016).
- Syntheses, structures, and NMR chemical shifts of a family of trimethyltin alkoxide, amide, halide and cyclopentadienyl compounds.: Details the synthesis and structural characterization of trimethyltin compounds, useful for understanding the coordination chemistry that may involve Tetrahydrofuran-d₈ as a solvent or structural analogue (Lichtscheidl et al., 2015).
- NMR studies of coupled low- and high-barrier hydrogen bonds in pyridoxal-5′-phosphate model systems in polar solution.: Discusses the application of NMR spectroscopy in studying hydrogen bonding interactions, where Tetrahydrofuran-d₈ could be utilized as a deuterated solvent for enhanced spectral clarity (Sharif et al., 2007).
- Stable hydrocarbon diradical, an analogue of trimethylenemethane.: Investigates stable hydrocarbon diradicals, where Tetrahydrofuran-d₈ may be used as part of the experimental setup to stabilize reactive intermediates or as a solvent to study radical stability (Rajca et al., 2005).
Recommended products
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service