240877
p-Toluenesulfonyl chloride
ReagentPlus®, ≥99%
Synonym(s):
TsCl, Tosyl chloride
About This Item
Recommended Products
vapor pressure
1 mmHg ( 88 °C)
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
solid
bp
134 °C/10 mmHg (lit.)
mp
65-69 °C (lit.)
solubility
benzene: freely soluble(lit.)
chloroform: freely soluble(lit.)
ethanol: freely soluble(lit.)
water: insoluble(lit.)
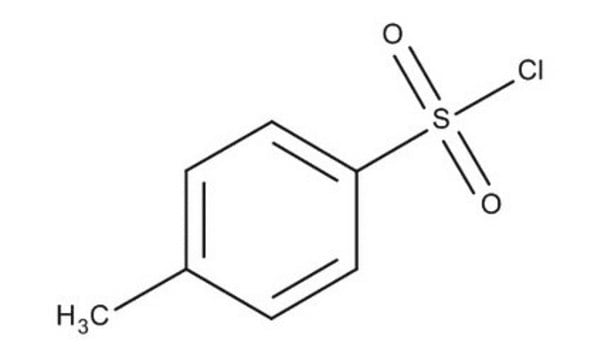
SMILES string
Cc1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H7ClO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3
InChI key
YYROPELSRYBVMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- In combination with N-methylimidazole for the esterification or thioesterification of carboxylic acids and alcohols or thiols.
- As an additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of arylboronic acids in the absence of ligands.
- As a positive chlorine source for the ?-chlorination of ketones.
- Solvent-free tosylation of alcohols and phenols in the presence of heterodoxy acids.
- As an activator for reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.
- As a catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
262.4 °F - closed cup
Flash Point(C)
128 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service