By substituting tetramethylated m-benzenediproprionic acid 1 via a decarboxylative metathesis of Rh2(OAc)4 (Scheme 1), the Du Bois group at Stanford has improved the catalytic performance of Rh with respect to the oxidative C–H activation of sulfamate, sulfamide, carbamate, urea, and guanidine substrates through nitrene insertion.1-2
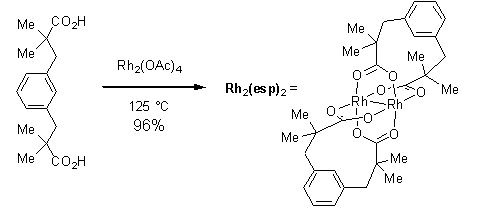
Scheme 1.Decarboxylative metathesis of Rh2(OAc)4
High yields of tri- and tetrasubstituted amines were found by conducting the reactions under slow addition of the oxidant PhI(O2CtBu)2; which is an easily prepared, stable, and unlike PhI(OAc)2, more soluble in non-polar solvents, effective oxidant for C-H amination. The proclivity of unactivated methylene groups to undergo C–H amination is a result of the catalyst’s robust nature. Substrates with 3° C–H bonds are fully converted to the desired heterocycle at catalyst loadings as low as 0.15 mol%. This exceptional improvement in catalyst turnover is illustrated in Scheme 2, wherein five times the amount of the isosteric Rh2(O2CtBu)4 must be applied in the intramolecular conversion of sulfamates to heterocycles to afford comparable product yields.
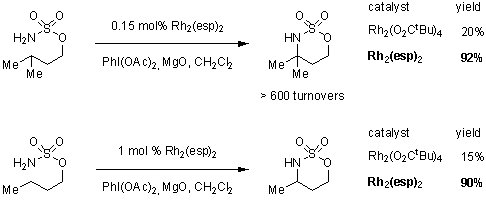
Scheme 2.
Du Bois and co-workers have also employed this highly efficient catalyst for the oxidative cyclization of sulfamide, urea, and guanidine substrates. Most impressively, Rh2(esp)2 has been found to facilitate the intermolecular C–H insertion into a range of benzylic and 3° substrates with 2,2,2-trichloroethylsulfamate as the N-atom source (Scheme 3). These reactions are generally performed with limiting amounts of starting material, a feature that distinguishes the Rh2(esp)2-catalyzed process from other intermolecular amination methods with Mn, Fe, Ru, and Cu. By employing this methodology, rapid access to amino alcohols, amino acids, and diamines is afforded.3-4
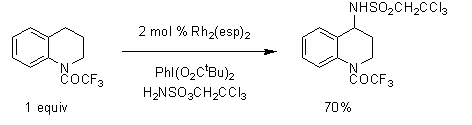
Scheme 3.
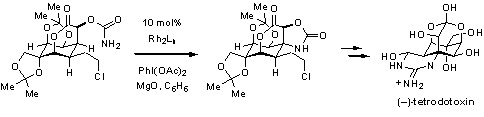
This new oxidative strategy offers the unique ability to control chemo-, regio-, and diastereoselective transformations for the preparation of 1,3-diamines, amino alcohols, and β-amino acids. The Du Bois group has developed this C–H amination for use in the total synthesis of (–)-tetrodotoxin (TTX), a highly efficacious blowfish poison.5 The densely functionalized, oxygenated cyclohexanone framework of TTX illustrates the difficult challenge in its attempted synthesis, of which only two successful strategies have been employed prior to this report.8-9 Stereospecific C–H bond amination using an appropriately configured 1° carbamate was used to install a C–N bond in asymmetric fashion at a late stage in the total synthesis of (–)-TTX (Scheme 4).10 These C–H activation methods and catalysts described by the Stanford group now offer a powerful tool for synthetic chemists to apply for the construction of functionalized amine frameworks.
References
To continue reading please sign in or create an account.
Don't Have An Account?