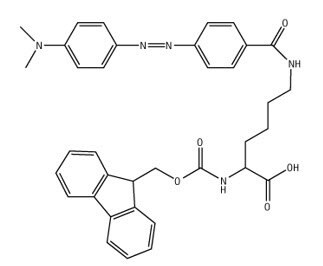
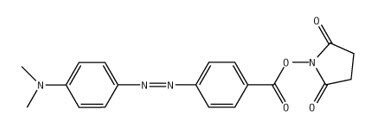
Peptide Labeling
Fluorescent- and biotin-labeled peptides are invaluable tools for biochemistry, having numerous applications in enzymology, protein chemistry, immunology and histochemistry. We offer an extensive range of labeling reagents for the synthesis of such peptides, including the unique NovaTag™ resins for the production of C-terminally-labeled peptides.
Chromogen labeling
The spectral properties of Novabiochem® chromogenic and fluorogenic derivatives are summarized in Table 1.
One of the most important applications of such reagents is in the synthesis of fluorescence-quenched enzyme substrates. These are highly sensitive tools for probing protease specificity and activity, particularly for endoproteases where conventional carboxy-terminally labeled substrates are not always appropriate. Such substrates typically contain a fluorophore and a quencher group attached to either side of the cleavage site. In the intact molecule, the natural fluorescence of the fluorophore is suppressed by the proximity of the quencher through a process called fluorescence resonance energy transfer (FRET). Upon cleavage of the substrate by a protease, the quencher and fluorophore become separated, leading to an increase in fluorescence, which can then be detected spectrophotometrically (Figure 1). Sensitivity is determined primarily by the distance between fluorophore and quencher, which should be in the range 10-100 Å, and the extent of overlap between the absorbance spectrum of the quencher and the emission spectrum of the fluorophore. The recommended fluorophore-quencher pairs are listed in Table 1.

Figure 1.Fluorescence-quenched peptide substrate

Figure 2. NovaTag™ resins
Labels are most frequently incorporated at the N-terminus of the peptide during solid phase synthesis as this is synthetically very straightforward using carboxylic acid derivatives of labels such as biotin-OSu, TAMRA, etc. For many applications, however, it is advantageous to place the label at the C-terminus, particularly if the N-terminus is required for biological activity, or if the peptide contains more than one label.
Furthermore, for FRET-based enzyme substrates, it is often preferable to place the fluorophore/quencher pair at the N- and C-termini, rather than on amino-acid side chains as this avoids modification of the native peptide sequence.
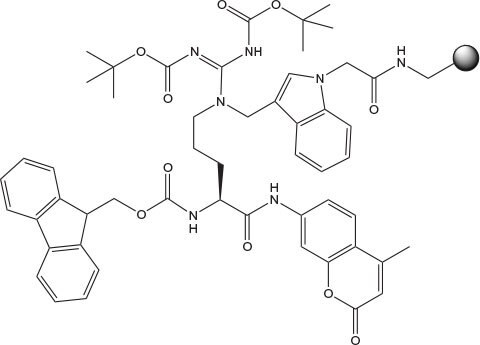
NovaTag™ resins are unique tools designed to streamline the Fmoc SPPS of chromogen and biotin labeled peptides1-3. Each resin contains a standard TFA-cleavable linker which has been modified to incorporate a diamine spacer that is either orthogonally protected or pre-derivatized with a chromogenic label (Figure 2). This arrangement allows C-terminally labeled peptides to be prepared quickly and efficiently with the minimum number of synthetic steps. Pre-loaded resins are available which on cleavage directly provide peptides containing fluorophores (Dansyl, Mca, EDANS) and quencher groups (Dnp) for FRET applications, or affinity labels (biotin, biotin-PEG, hydroxylamine) for bioconjugation and surface immobilization.
The pre-loaded resins are easy to use and can be generally employed in automated instrumentation without modification of existing Fmoc synthesis protocols. The only exceptions are the EDANS NovaTag™ and Universal NovaTag™ resins where attachment of the first residue must be carried out under modified conditions as this involves acylation of a secondary amine (Method 2). Following chain extension and cleavage with TFA in the usual manner, products are obtained containing the appropriate fluorophore or biotin at the C-terminus.
The use of pre-loaded resins avoids the inherent problems of poor solubility and inefficient coupling, as well as the additional steps and costs, that are associated with the introduction of biotin and certain fluorophores in solid phase synthesis. The approach is particularly suited to the production of peptides for high-throughput screening, as it removes the difficulty of ensuring complete label incorporation for all molecules in the array. NovaTag™ resins incorporating different labels and spacer groups can also be custom manufactured.

For situations where it is not always apparent at the outset which is the optimum label or combination of labels for a given application, we offer the Universal NovaTag™ resins. These supports facilitate the synthesis of peptides bearing any number of different acyl moieties at N- and C-termini from a single solid phase synthesis (Figure 3). Universal resins are also useful for preparing labeled peptides containing fluorophores that are not compatible with Fmoc SPPS protocols, such as TAMRA and FAM4, since they allow the labels to be easily introduced as the final step before cleavage. Universal PEG NovaTag™ resin is particularly efficacious for applications where a hydrophilic spacer is required between the peptide and the label and where the peptide solubility is an issue.
After loading (Method 2) of the first amino acid to the resin-bound secondary amine, chain extension is carried out under standard Fmoc methods. Following synthesis, the resin can be partitioned and each aliquot end-capped with the appropriate carboxyl-functionalized label. The pendant Mmt group is then removed using HOBt/TFE/DCM (Method 1) and the C-terminal label introduced to each resin aliquot. Thus, from a single synthesis any number of label variations for a given sequence can be prepared.
Universal PEG NovaTag™ resin has been used to prepare fluorescently-labled Shc Src homology 2 domain-binding peptides linked to cell penetrating sequences via a PEG spacer5 and dimeric SMAC PEG-linked peptides6. Universal NovaTag™ resin has been utilized to prepare peptides C-terminally modified to ketone for ligation7.
Method 1: Removal of Mmt group
- Add 0.6 M HOBt in DCM/TFE (1:1) to resin swollen in DCM.
- Gently agitate for 1h; solution goes dark red.
- The solvent is removed by filtration, and steps 1 & 2 are repeated.
- The resin is removed by filtration, washed with DMF and used immediately in synthesis, or washed further with DCM and then MeOH, dried and stored for later use.
Enzyme substrates based on the 7-amino-4-methylcoumarin (AMC) fluorophore are very popular tools for studying protease activity and specificity8. In such substrates, the AMC is typically linked to the peptide through formation of an amide bond between the coumarin amine and the carboxyl group of the C-terminal amino-acid residue (Figure 4). Proteolysis of this amide bond liberates free AMC, resulting in a large increase in fluorescence that can be detected at 441 nm upon excitation at 342 nm.
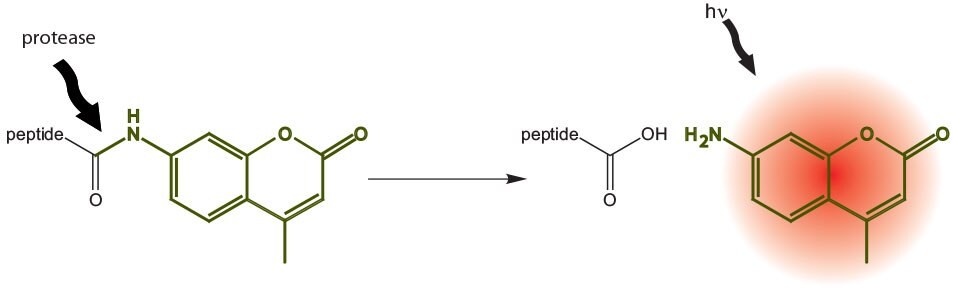
Figure 4.Principle of AMC-labeled fluorogenic substrates.
The synthesis of peptide-AMC derivatives is particularly problematic owing to the poor nucleophilicity of the AMC amine group. The usual strategy involves first formation of the AMC derivative of the C-terminal amino-acid residue followed by fragment condensation or stepwise elongation. This approach is obviously not amenable to solid phase methods and cannot be applied to the production of enzyme substrate libraries for protease profiling. To overcome these limitations, we have introduced pre-loaded with amino acid-AMC derivatives: Fmoc-Asp(Wang resin)-AMC; Fmoc-Lys(carbamate Wang resin)-AMC. Aspartic acid and lysine were selected as these amino acids occur at the P1 position of endogenous substrates for several important proteases.
These resins are extremely simple to use and are fully compatible with standard Fmoc SPPS protocols. The free amine group can be acylated with Fmoc amino acids activated with PyBOP® or TBTU. Following peptide assembly, cleavage with 95% TFA releases the peptide-AMC directly from the solid support without any additional steps.

Figure 5.Synthesis of Ac-Asp-Glu-Val-Asp-AMC using Fmoc-Asp(Wang resin)-AMC
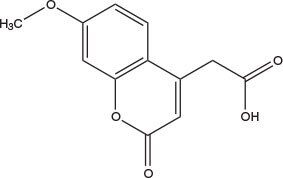
The 7-methoxycoumarin group (Mca) fluoresces at 393 nm when stimulated at 328 nm, and is most commonly used in conjunction with 2,4-dinitrophenyl (Dnp) quenching groups9. It can be coupled on solid phase to free amines using Mca-OSu, Mca-OH, or incorporated during chain extension using a pre-formed building block such as Fmoc-Lys(Mca)-OH10. Fmoc-Lys(Mca)-OH and Mca-OH can be coupled using any standard coupling method, such as PyBOP®/DIPEA and DIPCDI/HOBt, whereas the preactivated derivative, Mca-OSu, couples best in DMSO or NMP in the presence of HOBt. The coumarin moiety is stable to the standard conditions employed in Fmoc SPPS.
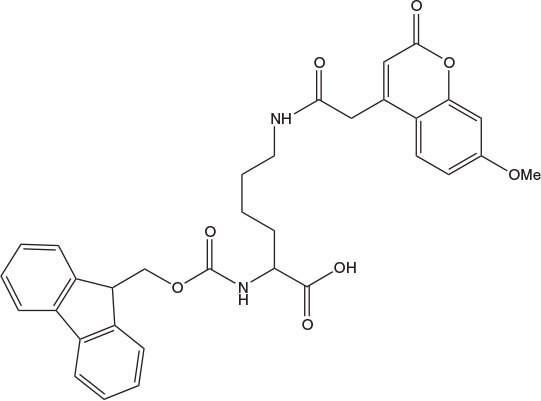
Mca NovaTag™ resin provides peptides C-terminally modified with the Mca fluorophore attached via an ethylene diamine spacer1-3. Following removal of the Fmoc group, the resin-bound primary amine can be loaded with the first amino acid residue using standard activation methods, such as PyBOP, HOBt/DIPCDI. After peptide assembly, treatment with 95% TFA cleaves the Mca peptide directly from the resin.
Dabcyl
Dabcyl is one of the most frequently utilized quenching groups because of its lack of innate fluorescence and spectral overlap (λmax 453 nm) with a number of commonly-used fluorophores, such as EDANS, Mca, TET, JOE, FAM. In the preparation of fluorescence-quenched peptide substrates, it is most frequently used in conjunction with EDANS as this pairing is particularly efficacious owing to their excellent spectral overlap11,12.
The Dabcyl group is most conveniently introduced during solid phase synthesis of the substrate. Addition to the N-terminal amino group is best achieved using Dabcyl-OSu in DMSO or NMP in the presence of HOBt. When the Dabcyl group is to be located in the peptide chain, the simplest approach is to introduce Lys(Dabcyl) at the desired position using Fmoc-Lys(Dabcyl)-OH activated with PyBOP®/DIPEA.
2,4-Dinitrophenyl (Dnp)
The Dnp group (λmax 348 nm) is the preferred quenching group for the Mca fluorophore9. It is most easily incorporated into a peptide as Fmoc-Lys(Dnp)-OH, which can be coupled using any standard activation method.
This resin is ideal for the assembly of labeled peptides incorporating a C-terminal Dnp group1-3. Following removal of the Fmoc group, the resin-bound primary amine can be loaded with the first amino acid residue using standard activation methods, such as PyBOP®, HOBt/DIPCDI. After peptide assembly, treatment with 95% TFA cleaves the Dnp peptide directly from the resin. This resin is particularly advantageous for the synthesis of FRET peptides, since the presence of the quencher in every peptide chain minimizes the levels of fluorescent unquenched by-products and so reduces background fluorescence of the product FRET substrates.
EDANS
Fmoc-Asp(EDANS)-OH and Fmoc-Glu(EDANS)-OH
The EDANS/Dabcyl fluorophore-quencher pair is one of the most commonly-used for FRET applications, owing to excellent spectral overlap between the emission spectrum of EDANS (λex 341 nm, λem 471 nm) and absorbance spectrum of Dabcyl (λmax 453 nm)9,10 (Figure 6). Quenching of the fluorescence of EDANS by Dabcyl is consequently highly efficient, with up to 40-fold enhancements in fluorescence having been observed upon proteolysis of Dabcyl/EDANS-labeled peptides13.
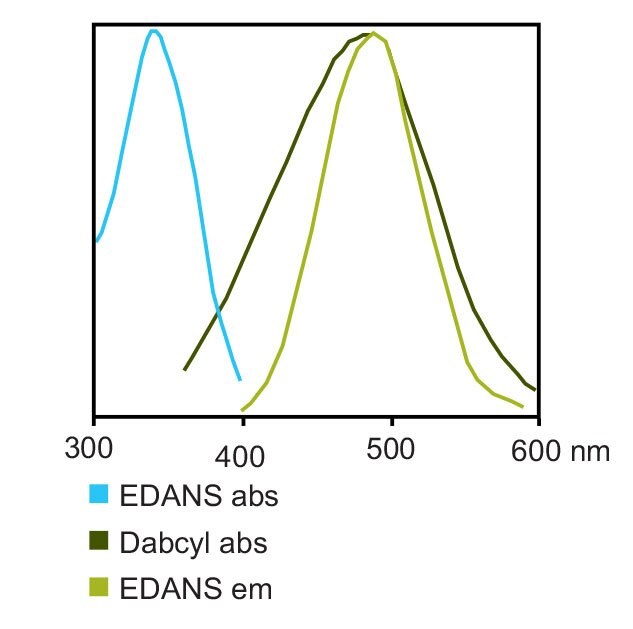
Figure 6.Absorbance and emission spectra of Dabcyl and EDANS
To incorporate EDANS within the peptide chain, the simplest approach is to use either Fmoc-Asp(EDANS)-OH or Fmoc-Glu(EDANS)-OH during peptide assembly13,14. Introduction of these derivatives during SPPS can be achieved using PyBOP®/DIPEA activation in conjunction with an extended coupling time14. Powerful acylating reagents such as PyBrOP should be avoided as their use may lead to acylation of the naphthylamine nitrogen.

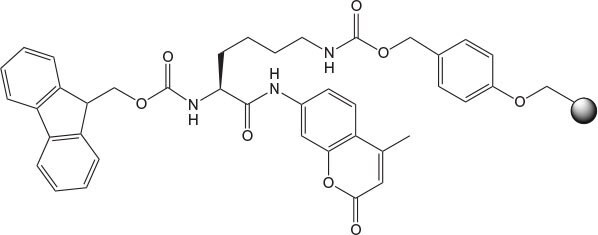
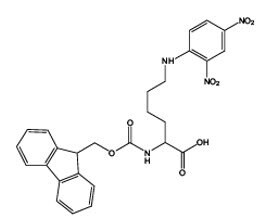
Figure 7.Loaded EDANS NovaTag™ resin showing point of attachment of peptide and site of cleavage.
Traditionally, the introduction of the EDANS moiety at the C-terminus of a peptide is achieved by coupling of a peptide fragment to EDANS in solution15. Novabiochem®’s EDANS NovaTag ™ resin enables for the first time the direct synthesis of C-terminally EDANS-labeled peptides by solid phase synthesis2 (Figure 7). Since the EDANS fluorophore is built into the linker, it becomes linked to the C-terminus of the peptide when the first amino acid is attached to the resin. The Dabcyl quencher group can be introduced to the N-terminus using Dabcyl-OSu in DMSO or DMF in the presence of HOBt. If the Dabcyl group is to be located in the peptide chain, the simplest approach is to introduce Lys(Dabcyl) at the desired position using Fmoc-Lys(Dabcyl)-OH activated with PyBOP®/DIPEA.
The first and most important step in using EDANS NovaTag™ resin is attachment of the first amino acid residue. As this process involves acylation of a resin-bound secondary amine, it is best carried out using HATU activation (Method 2). Pfp esters in the presence of collidine can also be employed, although longer acylation times may be required. It is important that this reaction is carried out to completion, as any unreacted amino groups left on the resin may react in subsequent couplings and lead to the formation of truncated sequences. Following loading, the substitution of the resin should be checked with the Fmoc UV assay, and if necessary, the loading reaction repeated using fresh reagents. Once loaded with the first amino acid, peptide synthesis can be carried out under standard conditions. The use of PyBroP® should be avoided as this can lead to double acylation. Cleavage from the resin can be effected using standard TFA cocktails; however, due to the proximity of the naphthylamine nitrogen to the cleavage site of the linker, product release can sometimes be sluggish. The reaction can be accelerated, if necessary, by the addition of a few drops of TMSBr to the standard TFA cocktail provided water is omitted.
EDANS NovaTag™ resin has been recently employed to prepare fluorescently labeled aminoalkane diphenyl phosphonate affinity probes for chymotrypsin- and elastase-like serine proteases16.
Method 2: Loading EDANS/Universal/Biotin-PEG NovaTag™ resins
- Suspend resin in DMF and leave to swell for 30 min. (In the case of Universal/Biotin-PEG NovaTag™ resin the Fmoc group should be removed at this stage with 20% piperidine in DMF.)
- Dissolve Fmoc-amino acid (2.5 eq.) and HATU (2.5 eq.) in minimum volume of DMF and add to resin. Add DIPEA (5 eq.) and mix.
- The mixture is left to stand for 2 h with gentle agitation. A sample of resin can be removed, and the loading determined using the Fmoc UV assay [Method 11: Estimation of level of first residue attachment]. Repeat the coupling with fresh reagents if necessary.
- The resin is removed by filtration, washed with DMF and used immediately in synthesis, or washed further with DCM and then MeOH, dried and stored for later use.
This resin facilitates the direct synthesis of peptides C-terminally labeled with the Dansyl group (λex 335 nm, λem 526 nm). Following removal of the Fmoc group, the resin-bound primary amine can be loaded with the first amino acid residue using standard activation methods, such as PyBOP®, HOBt/DIPCDI. After peptide assembly, treatment with 95% TFA cleaves the Dansyl peptide directly from the resin.
Dansyl NovaTag™ resin has been employed to prepare FRET probes for mercury binding protein MerP17 and functionalized amyloid fibrils18.
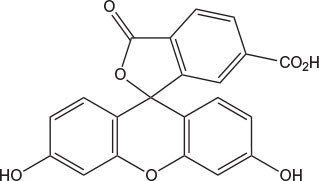
Novabiochem® supplies carboxyfluorescein (FAM; λex 494 nm, λem 518 nm) and carboxytetramethylrhodamine (TAMRA, λex 555 nm; λem 580 nm) as single isomers, ensuring labeled products of defined chemical structure, as well as greatly assisting product purification and characterization. However, for those applications which do not require a single isomer dye, 5,6-carboxyfluorescein is also available as the cost-effective option.
The dyes are most conveniently introduced during solid phase synthesis by coupling to N-terminal or side-chain amino groups using HOBt or HOAt/DIPCDI in DMF (Method 3). When one of the dyes is to be located on a side-chain amino group, the simplest approach is to incorporate an orthogonally-protected derivative, such as Lys(Mtt) or Lys(ivDde), that can be later selectively deprotected on the resin immediately prior to coupling of the dye. After addition of FAM and subsequent amino acids, the resin should be washed with 20% piperidine in DMF to remove phenyl esters formed by acylation of the FAM phenolic hydroxyls. Treatment of FAM peptides with hydrazine can result in hydrazone formation. Esters and hydrazone formation can both be avoided if, following the introduction of FAM and the subsequent piperidine treatment, the phenolic hydroxyls are blocked by tritylation with Trt-Cl and DIPEA in DCM2. TAMRA and FAM can also be added using pre-formed OSu esters.
When used together in the same peptide, fluorescence resonance energy transfer (FRET) between FAM and TAMRA results in quenching of the fluorescence of both dyes, making them excellent reagents for FRET applications19.
Method 3: Coupling of Carboxyl-functionalized Dyes
- Dissolve dye (1.5 eq.) in minimum DMF with HOBt or HOAt (1.5 eq.). If necessary, add DMSO to aid dissolution.
- Add DIPCDI (1.7 eq.) and stir mixture and leave to stand for 10 min.
- Add mixture of drained peptidyl resin and agitate gently for 2 h. Check for free amino groups using the Kaiser test. Note: the TNBS test does not work for beads loaded with colored dyes. If the reaction is not complete, leave o/n and then recheck. If after this time it is still not complete, wash resin and repeat with fresh reagents.
Atto Dyes
Atto dyes are a series of fluorescent dyes that meet the critical needs of modern fluorescent technologies:
- Stability - Atto 655 and Atto 647N are photostable and highly resistant to ozone degradation, making them ideal for microarray applications.
- Long Signal Lifetimes - Signal decay times of 0.6–4.1 nanoseconds allow timegate studies to reduce autofluorescence background and scattering.
- Reduced Background - Several Atto dyes have excitation wavelengths greater than 600 nm, reducing background fluorescence from samples, Rayleigh and Raman scattering.
- Selection - Atto dyes have strong fluorescent signals that cover visible and near-IR emission wavelengths.
Many of Atto dyes (Atto 590 and above) can be excited using wavelengths greater than 600 nm. Using long-wavelength activated Atto dyes in conjunction with the appropriate excitation wavelength reduces autofluorescence due to sample, solvent, glass, or polymer support, and improves overall sensitivity in biological analysis and imaging techniques. The background fluorescence due to Rayleigh and Raman scattering are also dramatically reduced by use of longer wavelength excitation.
Atto dyes have strong fluorescent signals with most having molar absorptivity values >100,000 and low excitation/emission overlap, making Atto dyes ideal for multiplex techniques using visible and near-IR emission wavelengths. With excitation signal maxima ranging from 390 to 740 nm and good Stokes shift separation, there are Atto dyes suitable for use with any common excitation light source.
References
To continue reading please sign in or create an account.
Don't Have An Account?