UHPLC Analysis of Nucleosides on Supel™ Carbon LC Column: Performance Comparisons with Competitor
William L. Maule III, Clint Corman, Michael Ye, Cory Muraco, Curtis Frantz
Merck Bellefonte, PA
Introduction
Nucleosides are an important class of small molecules that are the building blocks of nucleic acids. In addition, nucleosides have found interest as active pharmaceutical ingredients (APIs) in antiretroviral drugs and as biomarkers for certain diseases. However, due to the structural similarity between these analytes, developing an analytical method to characterize a series of these compounds can be challenging. This application demonstrates the use of the SupelTM Carbon LC column to resolve a set of 12 nucleosides.
Figure 1.Chemical Structures of Compounds Used in this Study.
| Experimental Conditions | |||
|---|---|---|---|
| Column(s) | Supel™ Carbon LC, 10 cm x 2.1 mm I.D., 2.7 µm | ||
| Competitor T, 10 cm x 2.1 mm I.D., 3.0 µm | |||
| Detection | UV; 260 nm | ||
| Buffer | 5 mM Ammonium formate in water, pH adjusted to 5.3 | ||
| Mobile phase | [A] 5 mM Ammonium formate, pH 5.3; [B] Acetonitrile | ||
| Gradient | Time (min) | %A | %B |
| 0 | 90 | 10 | |
| 40 | 40 | 60 | |
| 50 | 90 | 10 | |
| Test Mix | Nucleosides Test Mix, varied concentration, 1% sodium formate. | ||
| Injection Volume | 10 µL | ||
| Flow Rate | 0.25 mL/min | ||
| Temperature | Column: 55 °C | ||
| Test Mix Analyte/Concentration | |
|---|---|
| 1 | Cytidine (50 µg/mL) |
| 2 | Guanosine (25 µg/mL) |
| 3 | Inosine (25 µg/mL) |
| 4 | 1-Methyladenosine (25 µg/mL) |
| 5 | 5-Methylcytidine (100 µg/mL) |
| 6 | 2'-O-Methylcytidine (20 µg/mL) |
| 7 | 3-Methylcytidine methosulfate (100 µg/mL) |
| 8 | 7-Methylguanosine (25 µg/mL) |
| 9 | 5-Methyluridine (50 µg/mL) |
| 10 | ß-Pseudouridine (25 µg/mL) |
| 11 | 2-Thiocytidine dihydrate (10 µg/mL) |
| 12 | Uridine (25 µg/mL) |
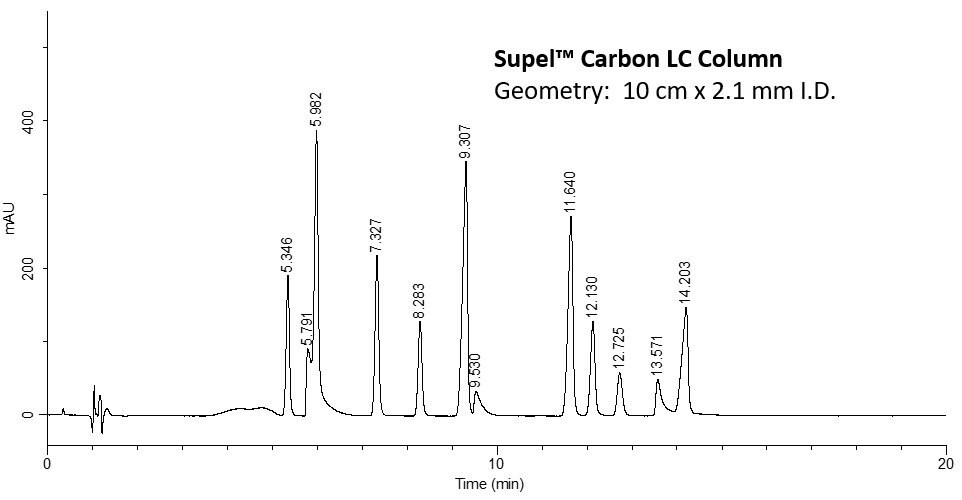
Figure 3.Analysis of Twelve Nucleosides on Supel™ Carbon LC.
| Peak | Compound | Retention Time (min) |
|---|---|---|
| 1 | ß-Pseudouridine (25 μg/mL) | 5.346 |
| 2 | 3-Methylcytidine methosulfate (100 µg/mL) | 5.791 |
| 3 | Cytidine (50 µg/mL) | 5.982 |
| 4 | Uridine (25 µg/mL) | 7.328 |
| 5 | 2'-O-Methylcytidine (20 µg/mL) | 8.283 |
| 6 | 5-Methylcytidine (100 µg/mL) | 9.307 |
| 7 | 1-Methyladenosine (25 µg/mL) | 9.530 |
| 8 | 5-Methylcytidine (100 µg/mL) | 11.641 |
| 9 | Inosine (25 µg/mL) | 12.130 |
| 10 | 7-Methylguanosine (25 µg/mL) | 12.725 |
| 11 | 2-Thiocytidine dihydrate (10 µg/mL) | 13.571 |
| 12 | Guanosine (25 µg/mL) | 14.203 |

Figure 4.Analysis of Twelve Nucleosides on a Competing Carbon Column.
| Peak | Compound | Ret. Time (min) |
|---|---|---|
| 1 | ß-Pseudouridine (25 µg/mL) | 5.242 |
| 2 | Cytidine (50 µg/mL) | 6.258 |
| 3 | Uridine (25 µg/mL) | 6.804 |
| 4 | 2'-O-Methylcytidine (20 µg/mL) | 8.652 |
| 5 | 5-Methylcytidine (100 µg/mL) | 9.705 |
| 6 | 5-Methyluridine (50 µg/mL) | 10.971 |
| 7 | 3-Methylcytidine methosulfate (100 µg/mL) | 11.616 |
| 8 | Inosine (25 µg/mL) | 12.199 |
| 9 | 2-Thiocytidine dihydrate (10 µg/mL) | 14.040 |
| 10 | 1-Methyladenosine (25 µg/mL) | N/A |
| 11 | Guanosine (25 µg/mL) | 17.366 |
| 12 | 7-Methylguanosine (25 µg/mL) | N/A |
Conclusion
This application has demonstrated the use of SupelTM Carbon LC column in resolving 12 nucleosides in under 15 minutes. The SupelTM Carbon LC column also outperforms a competitor column where two co-elutions occur and is an excellent choice for use in nucleoside biomarker identification and quantitation.
To continue reading please sign in or create an account.
Don't Have An Account?