M0252
Methylglyoxal solution
~40% in H2O
Synonym(s):
Acetylformaldehyde, Pyruvaldehyde, Pyruvic aldehyde
About This Item
Recommended Products
form
liquid
concentration
~40% (enzymatic)
~40% in H2O
density
1.17 g/mL at 25 °C (lit.)
storage temp.
2-8°C
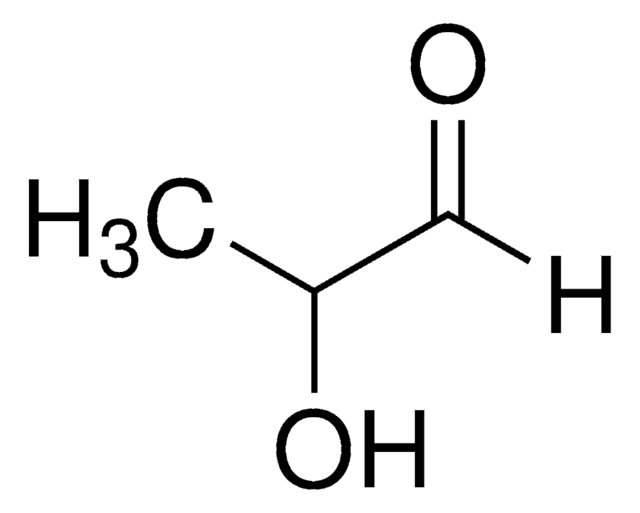
SMILES string
[H]C(=O)C(C)=O
InChI
1S/C3H4O2/c1-3(5)2-4/h2H,1H3
InChI key
AIJULSRZWUXGPQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to assess glyoxalase 1 (GLO1) enzymatic activity
- as an advanced glycation end (AGE) forming agent for the preparation of albumin in vitro
- to regulate anxiety like behavior in mice
- to induce peritoneal fibrosis in rats
- to study the chromatographic retention characteristics of organic chemicals and metal DNA adducts
- for intraplantar injection in mice to investigate peripheral and central components of methylglyoxal (MG)-transient receptor potential ankyrin 1 (TRPA1)-adenylyl cyclase 1 isoform (AC1) pathway
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Muta. 2 - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
High-Performance Thin-Layer chromatography (HPTLC) quantification of methylglyoxal (MGO) in complex and matrix rich manuka honey offering quick sample preparation, high-matrix tolerance, and high-throughput.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service