P1138
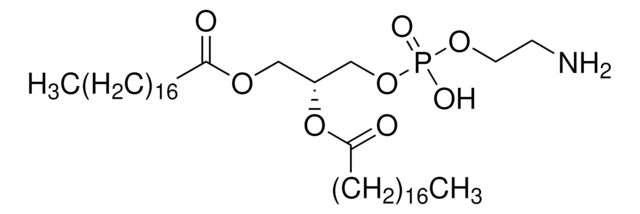
1,2-Distearoyl-sn-glycero-3-phosphocholine
≥99%
Synonym(s):
PC, 1,2-Dioctadecanoyl-sn-glycero-3-phosphocholine, 3-sn-Phosphatidylcholine, 1,2-distearoyl, L-α-Phosphatidylcholine, distearoyl, L-β,γ-Distearoyl-α-lecithin, DSPC, PC(18:0/18:0)
About This Item
Recommended Products
biological source
semisynthetic
Quality Level
Assay
≥99%
form
powder
functional group
phospholipid
lipid type
phosphoglycerides
shipped in
ambient
storage temp.
−20°C
SMILES string
[O-]P(OCC[N+](C)(C)C)(OC[C@]([H])(OC(CCCCCCCCCCCCCCCCC)=O)COC(CCCCCCCCCCCCCCCCC)=O)=O
InChI
1S/C44H88NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-43(46)50-40-42(41-52-54(48,49)51-39-38-45(3,4)5)53-44(47)37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h42H,6-41H2,1-5H3/t42-/m1/s1
InChI key
NRJAVPSFFCBXDT-HUESYALOSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Amantadine interactions with phase separated lipid membranes.: This study explores the interactions between amantadine and phase-separated lipid membranes, providing insights into the role of 1,2-Distearoyl-sn-glycero-3-phosphocholine in membrane structure and function (Kinnun et al., 2024).
- Design of charge converting lipid nanoparticles via a microfluidic coating technique.: This research designs lipid nanoparticles with charge-converting capabilities using 1,2-Distearoyl-sn-glycero-3-phosphocholine, enhancing drug delivery systems (Zöller et al., 2024).
- Investigation and Comparison of Active and Passive Encapsulation Methods for Loading Proteins into Liposomes.: The study compares methods for protein encapsulation into liposomes using 1,2-Distearoyl-sn-glycero-3-phosphocholine, advancing drug delivery technologies (Pisani et al., 2023).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service