Biotinylation Reagents
- Biotinylated Peptide Design
- AlphaScreen™ Protein-binding Assay
- Kinase Bind Assay
- Materials
- References
Biotin-labeled peptides have many important applications in immunology and histochemistry, such as affinity purification1 and FRET-based flow cytometry2, solid-phase immunoassays3, and receptor localization4, that exploit the high affinity of streptavidin and avidin for biotin.
Incorporation of the biotin label is best carried out during solid phase synthesis of the peptide ligand, with the optimum location for the biotin label dependent on the nature of the application.
The biotin label is most frequently located directly on the N-terminal group of the peptide, often without any regard to how this may affect peptide-target interactions, biotin-avidin binding, and the solubility properties of the resultant peptide. In many instances the products are poorly soluble and have little biological activity and poor affinity for biotin. Problems can also arise during the synthesis of such N-terminally biotinylated peptides due to the poor solubility and reactivity of many of the reagents used for biotin introduction.
Novabiochem®’s biotin-loaded NovaTag™ resins provide a simple and elegant solution to these problems5-9. Using these resins, biotinylated peptides are obtained directly following TFA cleavage, without the need for any additional biotinylation steps. Resins incorporate either an ethylenediamine or a 15 atom PEG spacer between the peptide and biotin to reduce steric hindrance.
The use of Fmoc-PEG Biotin NovaTag™ resin is particularly advantageous because not only does the hydrophilic PEG chain confer better solubility to the peptide biotin conjugate, but its extended conformation leads to better avidin binding which can dramatically improve assay sensitivity as demonstrated in Figure 2. Since the biotin is an integral part of the linker, its presence in every peptide chain is assured from the outset.
Fmoc-PEG Biotin NovaTag™ resin can be used directly in an automated synthesizer in the same manner as Rink amide resin. The Fmoc group is removed with 20% piperidine and the peptide assembled on the support using standard protocols. Cleavage from the resin using standard TFA cocktails affords the C-terminally labeled biotinylated peptide.
Biotinylated peptide design
When designing biotinylated peptides for use in assays, two of the most important considerations are the position of the biotin moiety and the nature of the spacer group between the peptide and biotin. These can profoundly affect the strength of peptide-protein and biotin-avidin interactions and consequently the sensitivity of the assay. The importance of correct peptide presentation is illustrated in the following examples taken from developmental work on protein-binding and kinase assays carried out at Merck KGaA, Darmstadt, Germany10.
AlphaScreen™ protein-binding assay
The peptide-protein binding assay was conducted using the AlphaScreen™ technology as shown in Figure 1. N- and C-terminally biotin-labeled versions of the native peptide ligand immobilized on streptavidin-coated donor beads were screened against acceptor beads loaded with target protein. Only the peptide which was C-terminally labeled with PEG-biotin had acceptable solubility in the test buffer and showed significant levels of protein binding (Figure 2).
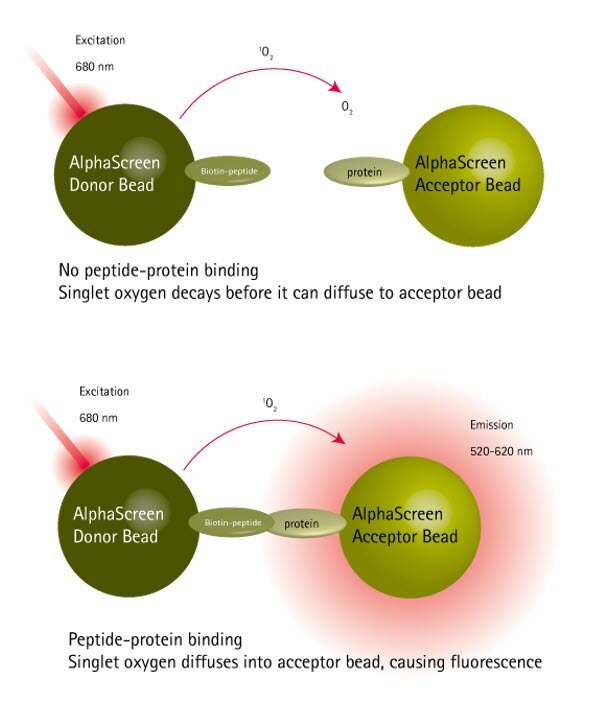
Figure 1.Principles of the protein-peptide binding AlphaScreen™ assay.
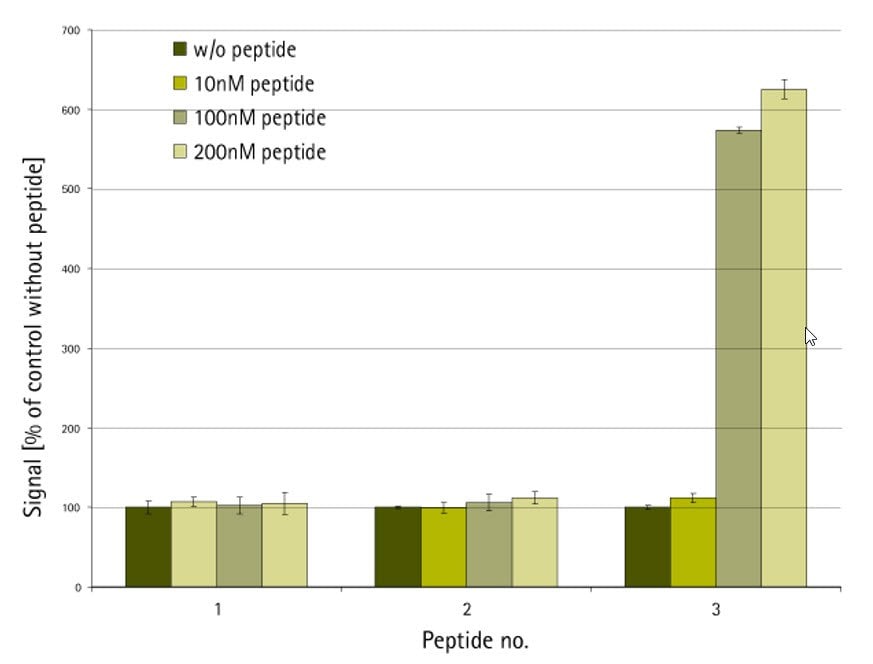
Figure 2.AlphaScreen protein-binding assay. Peptide 1: N-biotin-XXXXX-NH2; peptide 2: H-XXXXX-NHCH2CH2NH-biotin; peptide 3: H-XXXXX-NH-PEG-NH-biotin10
Kinase bind assay
N- and C-terminally biotin-labeled versions of a kinase substrate were evaluated in the assay shown in Figure 3. Peptides that were C-terminally labeled with biotin were found to give better reponses than those that were labeled on the N-terminus, whilst inclusion of a PEG spacer between the peptide and biotin appeared to have little effect (Figure 4).
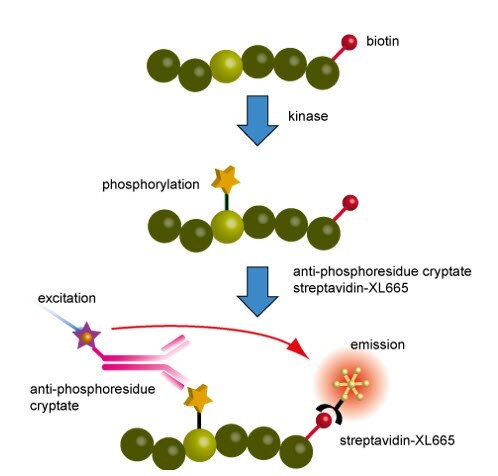
Figure 3.Principles of the kinase assay
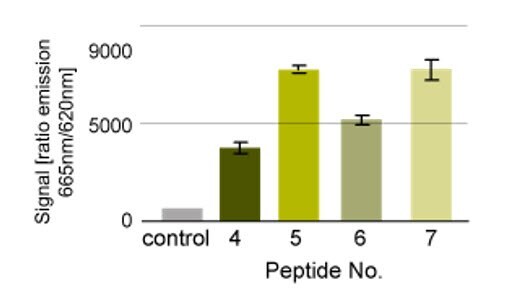
Figure 4.Kinase assay. Peptide 4: biotin-KKKKXXLLDXXXXXXXXXMKDEE-NH2; peptide 5: H-KKKKXXLLDXXXXXXXXXMKDEE-NHCH2CH2NH-biotin; peptide 6; biotin-NH-PEG-KKKKXXLLDXXXXXXXXXMKDEE-NH2 ; peptide 7: H-KKKKXXLLDXXXXXXXXXMKDEE-NH-PEG-NH-biotin10.
For coupling of biotin to amines on the solid phase the use of Biotin-ONp is strongly recommended11. The solubility of this reagent in DMF or NMP is much greater than that of Biotin-OSu (Table 1), and it couples with amines much more rapidly (Figure 5): typically, in 40 min as opposed to 12 h for Biotin-OSu.
| Solubility (mmole/mL) | |||
|---|---|---|---|
| Compound | DCM | DMF | NMP |
| Biotin-OSu | >0.02 | 0.1 | 0.15 |
| Biotin-ONp | 0.09 | 0.5 | 0.5 |
| Fmoc-Lys(biotin)-OH | <0.025 | <0.05 | 0.25 |
| Fmoc-Lys(biotinyl-e-aminocaproyl)-OH | <0.025 | 0.5 | 0.5 |
| SFmoc-Glu(biotinyl-PEG)-OH | <0.025 | 0.5 | 0.5 |
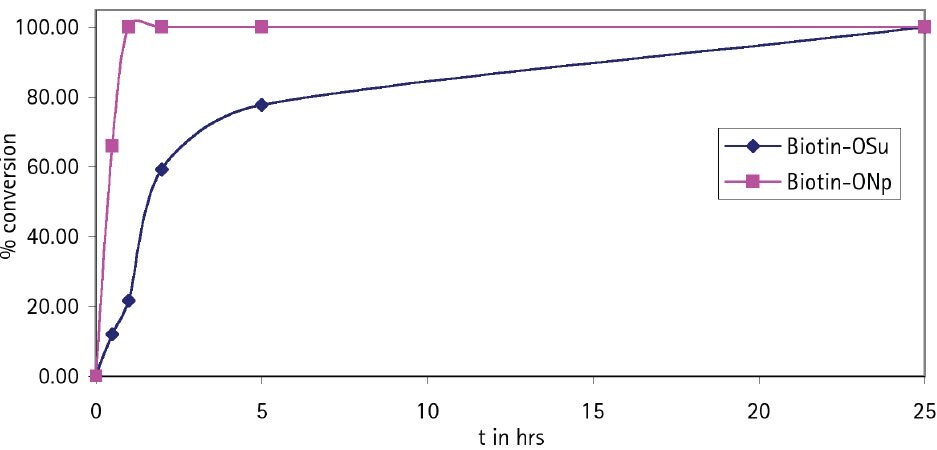
Figure 5.Coupling rate of Biotin-OSu and Biotin-ONp to H-Asp(OtBu)-Glu(OtBu)-Val-Glu(OtBu)-Wang resin.
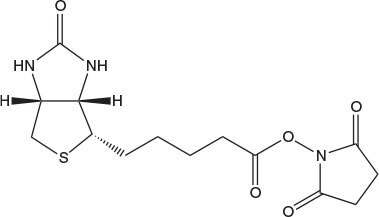
Alternatively, preformed derivatives such as Fmoc-Lys(biotin)-OH, Fmoc-Lys(biotinyl-e-aminocaproyl)-OH, Fmoc-Asp(biotinyl-PEG)-OH and Fmoc-Glu(biotinyl-PEG)-OH can be used to introduce biotin at a precise location within a peptide chain. The use of Fmoc-Glu(biotinyl-PEG)-OH is particularly recommended. In contrast to the other derivatives, it possesses good solubility in DMF (Fmoc-Glu(biotinyl-PEG)-OH, 0.5 mmole/ml; Fmoc-Lys(biotin)-OH, <0.05 mole/ml; Fmoc-Lys(biotinyl-e-aminocaproyl)-OH, <0.05 mmole/ml). Furthermore, the PEG chain improves the solubility of the product biotinylated peptide [1b] and helps reduce steric hindrance between peptide and biotin, leading to better avidin binding. It is recommended that NMP or NMP/DMSO be used as the solvents for Fmoc-Lys(biotin)-OH and Fmoc-Lys(biotinyl-e-aminocaproyl)-OH. For convenience, Fmoc-Lys(biotinyl-e-aminocaproyl)-OH pre-loaded onto NovaSyn® TGR A resin is available.
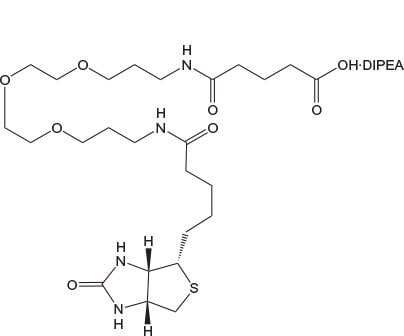
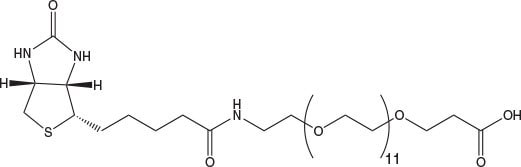
N-Biotinyl-NH-PEG2-COOH and N-Biotinyl-NH-PEG11-COOH offer the same benefits as Fmoc-Glu(biotinyl-PEG)-OH and are ideal for incorporation of biotin-PEG at the N-terminus of a peptide. The long 40 atom spacer in the latter has a marked effect on peptide solubility compared to that of Glu(biotinyl-PEG) or N-biotinyl-NH-PEG.
References
To continue reading please sign in or create an account.
Don't Have An Account?

-oh/fmoc-lys(biotin)-oh.jpg)
-oh/fmoc-lys(e-capbiotin)-oh.jpg)
-oh/Fmoc-asp(PEG-biotin)-oh.jpg)
-oh/Fmoc-glu(PEG-biotin)-oh.jpg)