T7140
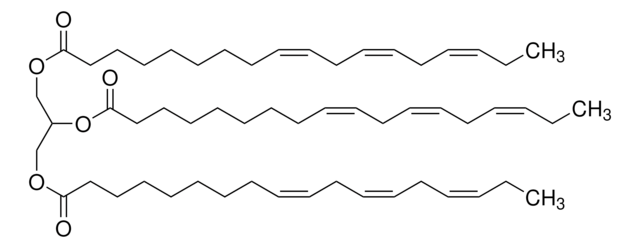
Glyceryl trioleate
≥99%
Synonym(s):
(9Z)9-Octadecenoic acid 1,2,3-propanetriyl ester, 1,2,3-Tri(cis-9-octadecenoyl)glycerol, Glycerol trioleate, Glycerol triolein, Oleic acid triglyceride, Oleic triglyceride, TG(18:1(9Z)/18:1(9Z)/18:1(9Z)), Triolein
About This Item
Recommended Products
biological source
plant (sunflower)
Assay
≥99%
form
oil
bp
235-240 °C/18 mmHg (lit.)
density
0.91 g/mL (lit.)
functional group
ester
lipid type
neutral glycerides
shipped in
ambient
storage temp.
−20°C
SMILES string
[H]C(COC(CCCCCCC/C=C\CCCCCCCC)=O)(OC(CCCCCCC/C=C\CCCCCCCC)=O)COC(CCCCCCC/C=C\CCCCCCCC)=O
InChI
1S/C57H104O6/c1-4-7-10-13-16-19-22-25-28-31-34-37-40-43-46-49-55(58)61-52-54(63-57(60)51-48-45-42-39-36-33-30-27-24-21-18-15-12-9-6-3)53-62-56(59)50-47-44-41-38-35-32-29-26-23-20-17-14-11-8-5-2/h25-30,54H,4-24,31-53H2,1-3H3/b28-25-,29-26-,30-27-
InChI key
PHYFQTYBJUILEZ-IUPFWZBJSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as an experimental diet along with fat-free basal mix and corn oil and then to access the dietary fat absorption among mice
- as an interfering substance to test its effect on human serum in the approach to develop rapid enzyme immunoassay for the detection of retinol-binding protein
- as a standard in the determination of triglyceride concentration, colorimetrically using liver tissue sample from cows
Packaging
Storage Class Code
10 - Combustible liquids
WGK
awg
Flash Point(F)
626.0 °F - closed cup
Flash Point(C)
330.0 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service