1151855
USP
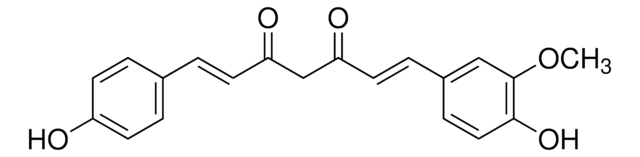
Curcumin
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
(E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Diferuloylmethane, Diferulylmethane, Natural Yellow 3
About This Item
Recommended Products
grade
pharmaceutical primary standard
vapor density
13 (vs air)
API family
curcumin
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O
InChI
1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+
InChI key
VFLDPWHFBUODDF-FCXRPNKRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Evaluating the dose-dependent effects of curcumin nano-micelles on rumen fermentation, nitrogen metabolism, and nutrient digestibility in heat-stressed fattening lambs: This study investigates the application of high-purity curcumin in improving animal health and productivity under climate stress, highlighting its potential as a bioactive compound in veterinary nutritional research (Bokharaeian et al., 2024).
- Curcumin reduces myocardial ischemia-reperfusion injury, by increasing endogenous H2S levels and further modulating m6A: This research underscores curcumin′s bioactive role in cardiovascular protection, offering insights into its mechanism of action within cell signaling pathways related to heart health (Cui et al., 2024).
- Targeting CD8+ T cells with natural products for tumor therapy: Revealing insights into the mechanisms: This review discusses the therapeutic applications of curcumin in cancer treatment, focusing on its interaction with cellular mechanisms and its potential as a molecular marker in immunotherapy (Wang et al., 2024).
- Senolytic effects on dental pulp stem cell′s proliferation and differentiation during long-term expansion: This article examines curcumin′s potential to influence cell growth and repair processes, pertinent to its research applications in dental and regenerative medicine (Wang et al., 2024).
- Future aspects of plant derived bioactive metabolites as therapeutics to combat benign prostatic hyperplasia: This review highlights curcumin′s role as a leading natural compound in pharmacological research, focusing on its anti-inflammatory and neuroprotective properties for treating conditions like benign prostatic hyperplasia (Krishnamoorthi et al., 2024).
Biochem/physiol Actions
Analysis Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service