345857
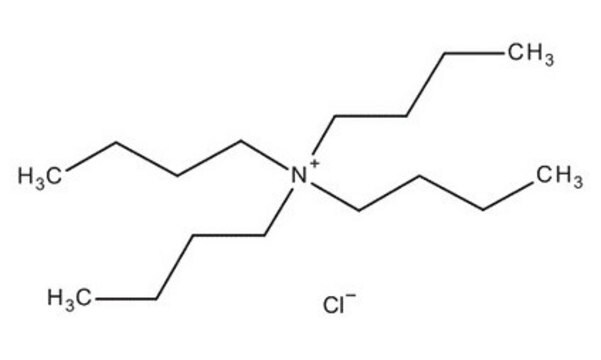
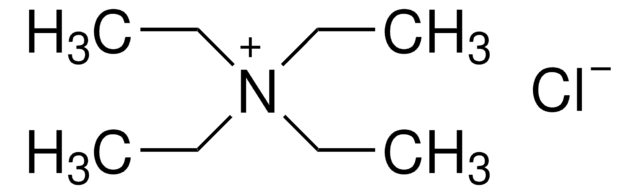
Tetrabutylammonium chloride hydrate
98%
Synonym(s):
TBAC hydrate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3CH2CH2CH2)4N(Cl) · xH2O
CAS Number:
Molecular Weight:
277.92
Beilstein:
3765327
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
41-44 °C (lit.)
InChI
1S/C16H36N.ClH.H2O/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;;/h5-16H2,1-4H3;1H;1H2/q+1;;/p-1
InChI key
FODWRUPJCKASBN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Tetrabutylammonium chloride (TBAC) hydrate has been used as a phase transfer catalyst in the synthesis of fullerenols and in Heck-type palladium-catalyzed vinylation of organic halides in water. TBAC hydrate has also been reported to be a promising candidate for cool energy storage material.
Tetrabutylammonium chloride hydrate can be used:
- As an additive in palladium-catalyzed cross-coupling reaction of heteroaromatic carboxylic acids with aryl bromides.
- To enhance the reactivity of palladium-catalyzed arylation of methyl acrylates.
- In derivatization of bis(spirodienone) calixarene having exocyclic double bonds to its chloro derivative.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Unexpected intermolecular Pd-catalyzed cross-coupling reaction employing heteroaromatic carboxylic acids as coupling partners
Forgione P, et al.
Journal of the American Chemical Society, 128(35), 11350-11351 (2006)
Phase equilibrium of ionic semiclathrate hydrates formed with tetrabutylammonium bromide and tetrabutylammonium chloride.
Sato K, et al.
Fluid Phase Equilibria, 337, 115-118 (2013)
Heck-type reactions in water.
Jeffery T, et al.
Tetrahedron Letters, 35(19), 3051-3054 (1994)
Electrical and optical properties of fullerenol Langmuir? Blodgett films deposited on polyaniline substrates.
Rincon M E, et al.
The Journal of Physical Chemistry B, 107(17), 4111-4117 (2003)
On the efficiency of tetraalkylammonium salts in Heck type reactions
Jeffery T
Tetrahedron, 52(30), 10113-10130 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service