All Photos(1)
About This Item
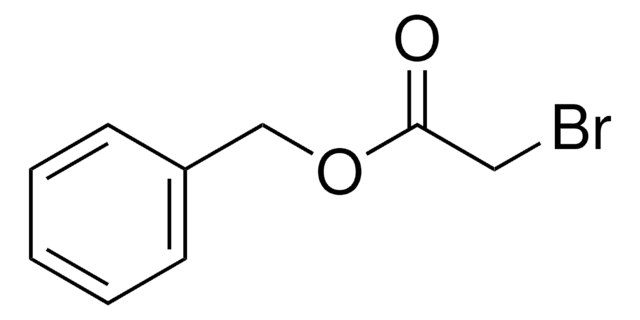
Linear Formula:
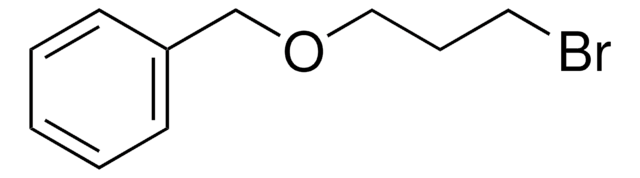
C6H5CH2OCH2CH2Br
CAS Number:
Molecular Weight:
215.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.541 (lit.)
bp
254 °C (lit.)
density
1.36 g/mL at 25 °C (lit.)
SMILES string
BrCCOCc1ccccc1
InChI
1S/C9H11BrO/c10-6-7-11-8-9-4-2-1-3-5-9/h1-5H,6-8H2
InChI key
FWOHDAGPWDEWIB-UHFFFAOYSA-N
General description
Benzyl 2-bromoethyl ether is an organic building block. Its enthalpy of vaporization at boiling point (527.15K) has been reported to be 46.499kjoule/mol.
Application
Alkylation reagent for synthesis of a macrocyclic antifungal antibiotic.
Building block used to prepare a copper phthalocyanine discotic liquid crystal.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>235.4 °F - closed cup
Flash Point(C)
> 113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fodil Bouazza et al.
Organic letters, 5(22), 4049-4052 (2003-10-24)
[reaction: see text]. (-)-PF1163B, a new macrocyclic antifungal antibiotic isolated from Streptomyces sp., has been prepared in eight steps from (S)-citronellene. The key step is a ring-closing metathesis reaction of an ester and amide derivative obtained from a substituted N-methyl-l-tyrosine.
680290
CorpBase ID (for auto-filling citation data) null
Chemistry of Materials, 17, 1618-1618 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service