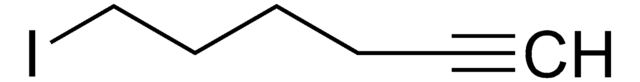
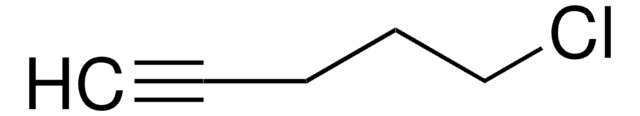
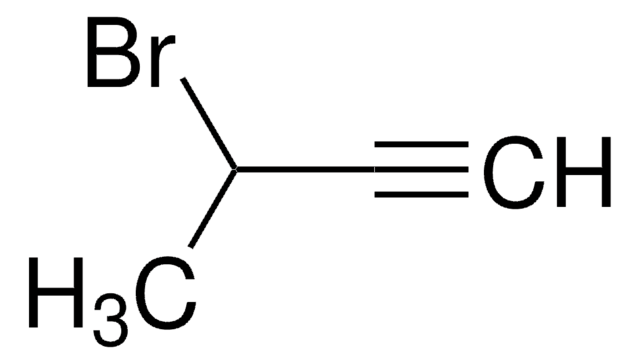
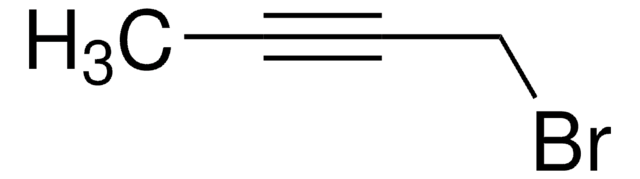
675725
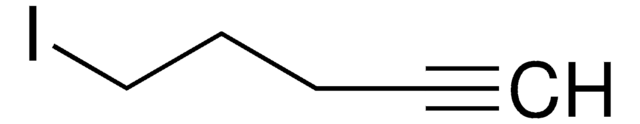
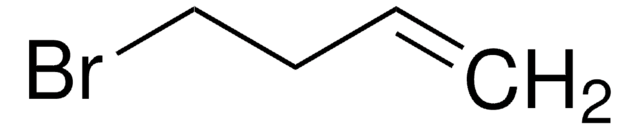
4-Bromo-1-butyne
97%
Synonym(s):
1-Bromo-3-butyne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5Br
CAS Number:
Molecular Weight:
132.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.481
density
1.417 g/mL at 25 °C
SMILES string
BrCCC#C
InChI
1S/C4H5Br/c1-2-3-4-5/h1H,3-4H2
InChI key
XLYOGWXIKVUXCL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Bromo-1-butyne is commonly used as a reactant. It serves as a source of alkyl halides for the introduction of bromo functionality into the molecule.
Application
4-Bromo-1-butyne is used as a reactant in the synthesis of:
- Macrocycles by cobalt-mediated [2+2+2] co-cyclotrimerization.
- 2,4,5-trisubstituted oxazoles by a gold-catalyzed formal [3+2] cycloaddition.
- Intramolecular 1,3-dipolar cycloaddition to synthesize 1,3,4-oxadiazoles.
- Lactones bearing alkynes for reductive cyclization in the preparation of azulene derivatives.
- Substituted α-pyrones by gold-catalyzed coupling reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
75.0 °F
Flash Point(C)
23.9 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A facile synthesis of N-C linked 1, 2, 3-triazole-oligomers
V Fiandanese, et al.
Tetrahedron, 67, 5254-5260 (2011)
Synthesis of macrocycles via cobalt-mediated [2+ 2+ 2] cycloadditions.
Bonaga LVR, et al.
Journal of the American Chemical Society, 127(10), 3473-3485 (2005)
Intramolecular Diels- Alder/1, 3-dipolar cycloaddition cascade of 1, 3, 4-oxadiazoles.
Elliott GI, et al.
Journal of the American Chemical Society, 128(32), 10589-10595 (2006)
Intermolecular and Selective Synthesis of 2, 4, 5-Trisubstituted Oxazoles by a Gold-Catalyzed Formal [3+ 2] Cycloaddition.
Davies PW, et al.
Angewandte Chemie (International Edition in English), 50(38), 8931-8935 (2011)
Reductive Cyclization Cascades of Lactones Using SmI2- H2O.
Parmar D, et al.
Journal of the American Chemical Society, 133(8), 2418-2420 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service