342001
Procathepsin K, Human, Recombinant, E. coli
Recombinant, human procathepsin K expressed in E. coli with a methionine residue inserted at amino acid 18 to create a new N-terminal initiation site.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
Assay
≥95% (SDS-PAGE)
form
liquid
specific activity
≥1000 mU/mg protein
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
avoid repeated freeze/thaw cycles
shipped in
wet ice
storage temp.
−70°C
General description
If the activated enzyme is not used immediately, it is recommended to add methyl methanthiosulfonate (1 mM final concentration MMTS).
Note: 1 mU = 1 milliunit.
Recombinant, human procathepsin K (amino acids 19-329) (GenBank target symbol = S79895, ACC No. P43235) expressed in E. coli with a methionine residue inserted at amino acid 18 to create a new N-terminal initiation site. Cathepsin K, a member of the papain superfamiliy of cysteine proteinases, plays an important role in osteoclast-mediated bone resorption and collagen degradation. Cathepsin K is synthesized as an inactive proenzyme that is converted to its mature, active form by proteolytic cleavage of the 99 amino acid propeptide domain. Inhibitors of cathepsin K include leupeptin (Cat. No. 108975) (IC50 = 70 nM), E-64 (Cat. No. 324890) (IC50 = 5 nM), and cystatin (Cat. No. 324891 or 324896). Requires activation prior to use.
Recombinant, human procathepsin K (amino acids 19-329) (GenBank target symbol = S79895, ACC No. P43235) expressed in E. coli with a methionine residue inserted at amino acid 18 to create a new N-terminal initiation site. Cathepsin K, a member of the papain superfamily of cysteine proteinases, plays an important role in osteoclast-mediated bone resorption and collagen degradation. Cathepsin K is synthesized as an inactive proenzyme that is converted to its mature, active form by proteolytic cleavage of the 99 amino acid propeptide domain. Inhibitors of cathepsin K include leupeptin (Cat. No. 108975) (IC50 = 70 nM), E-64 (Cat. No. 324890) (IC50 = 5 nM), and cystatin (Cat. No. 324891 or 324896). Requires activation prior to use.
Packaging
Please refer to vial label for lot-specific concentration.
Warning
Toxicity: Standard Handling (A)
Unit Definition
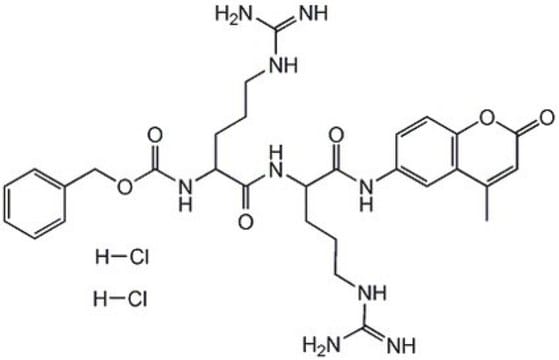
One unit is defined as the amount of enzyme that will hydrolyze 1 µmole benzyloxycarbonyl-phenylalanine-arginine-7-amido-4-methylcoumarin per min at 37°C, pH 5.5.
Physical form
In 500 mM NaCl, 25 mM Tris, pH 8.0.
Reconstitution
Following initial thaw, aliquot and freeze (-70°C). Following activation the enzyme is unstable and should include MMTS for storage (see recommended reaction conditions for activation).
Other Notes
McQueney, M., et al. 1997. J. Bio. Chem.272, 13955.
Bossard, M., et al. 1996. J. Biol. Chem.271, 12517.
Drake, F., et al. 1996. J. Biol. Chem.271, 12511.
Bromme, D. and Okamoto, K. 1995. Biol. Chem. Hoppe-Seyler376, 379.
Baron, R. 1989 Anat. Rec.224, 317.
Littlewood-Evans, A.J., et al. 1975. Cancer Res.57, 5386.
Nishimura, J.S. et al. 1975. Arch. Biochem. Biophys.170, 461.
Bossard, M., et al. 1996. J. Biol. Chem.271, 12517.
Drake, F., et al. 1996. J. Biol. Chem.271, 12511.
Bromme, D. and Okamoto, K. 1995. Biol. Chem. Hoppe-Seyler376, 379.
Baron, R. 1989 Anat. Rec.224, 317.
Littlewood-Evans, A.J., et al. 1975. Cancer Res.57, 5386.
Nishimura, J.S. et al. 1975. Arch. Biochem. Biophys.170, 461.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service