M-Trace® Electronic Test Record Software
The M-Trace® Software & Mobile App is your 21 CFR Part 11 compliant Digital QC companion for Sterility Testing, for trusted and efficient data traceability! Watch the videos to learn more, and see how a fully traceable, reliable and efficient sterility test performed with the assistance of the M-Trace® software could look like at your site.
Get introduced to your M-Trace® digital QC companion
Experience a sterility test using the M-Trace® solution
Filling the gap in Sterility Testing: Traceability and 21 CFR Part 11 Compliance
By giving step-by-step guidance and recording data automatically in realtime, the M-Trace® system offers a convenient and reliable way to comply with regulatory requirements regarding traceability and data integrity.
- Connect the M-Trace® software to your current Steritest® Symbio pump(s)
- Run the software from any computer using your centralized user management for Microsoft Windows
- Benefit from enhanced 21 CFR part 11 compliance thanks to the audit trail function
- Ensure full traceability and regulatory compliance from real-time, automatic, and complete recording of data for each of your sterility tests
- Increase process safety with guided workflows and deviation alerts
- Improve efficiency by eliminating paper-based documentation and digitizing recording and review processes
- Facilitate automated control of your Steritest® Symbio pump(s) and greater connectivity of your testing data to your lab IT environment (one single software to control all your pumps)
- Simplify training of operators with comprehensive real time instructions
Get full control of your entire Sterility Testing workflow
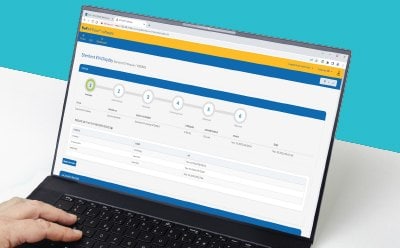

Connect Data From Anywhere
Connect information on samples, consumables, test points or devices, and test results from the lab or anywhere with the M-Trace® Mobile App.

Validate any Method Step (e.g. using voice control function)
Confirm the proper execution of a work step either by voice control or by machine-to-machine communication to allow real time documentation while your hands are busy with other tasks.
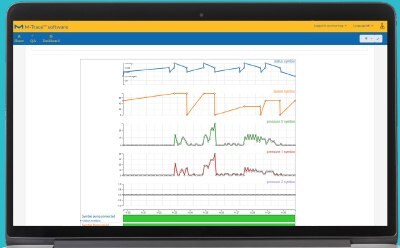
Monitor Your Process
Obtain instrument parameters such as pump speed or pressure from instruments such as the Steritest® Symbio pump.
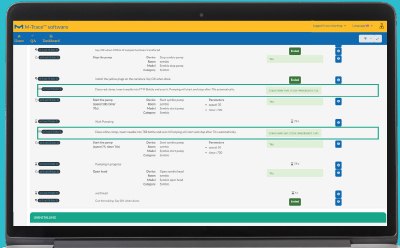
Generate Comprehensive Reports
A comprehensive report is automatically created containing all information on consumables used, incidents that occurred, and confirmation that each work step has been carried out as defined.
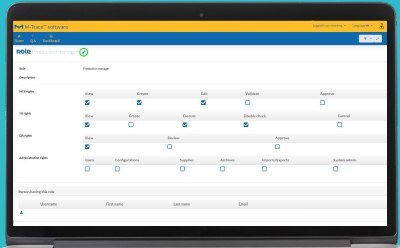
Ensure Compliance
Define roles and user rights in the comprehensive and convenient 21 CFR part 11 compliant user management system.
Related Product Resources
Get more detailed information and learn how a fully traceable, reliable and efficient sterility test performed with the assistance of our M-Trace® software could look like at your site.
Get additional technical information about your digital QC companion for sterility testing and other regulated processes.
Get access to the detailed analysis related to 21 CFR Part 11 compliance of our M-Trace® software.
Related Webinars
Listen to our experts and explore how you could benefit from using our M-Trace®solution.
Learn how innovative technologies and digitalized solutions can address data integrity challenges in microbiology Quality Control Labs.
To continue reading please sign in or create an account.
Don't Have An Account?



