Famciclovir Tablets USP Monograph Method Using a Purospher™ STAR RP-8 endcapped HPLC Column and UV Detection
Sonal Shinde, Application Specialist
Introduction
Famciclovir is an antiviral drug indicated for the treatment of herpes zoster, herpes simplex virus 2 (genital herpes), herpes labialis (cold sores), etc. It is a guanosine analogue, a prodrug form of penciclovir, and marketed by Novartis under the trade name Famvir. Generics are produced by TEVA and Mylan, among others. Purospher™ STAR RP-8 endcapped HPLC columns can be used to monitor organic impurities in tablet formulations following the new USP monograph for Famciclovir Tablets. The method suitability requirements are defined by the relative standard deviation (NMT 5.0% for Famciclovir standard solution) and the chromatographic resolution between propionyl famciclovir and 6-chloro famciclovir (NLT 1.2 using the system suitability solution).
| HPLC Conditions | |
|---|---|
| Column | Purospher™ STAR RP-8 endcapped (5 µm) 250x4.6 mm (1.51454) |
| Mobile Phase | [A] 2.72 g/L of monobasic potassium phosphate in water. Adjust with 1 M phosphoric acid to a pH of 4.0 ±0.05. |
| [B] Acetonitrile | |
| Gradient | See table |
| Flow Rate | 1 mL/min |
| Pressure Drop | 132-147 bar (1914-2131 psi) |
| Detection | UV @ 220 nm (analytical flow cell; 10 µL) |
| Temperatures | Column: 50 °C; Autosampler: 8 °C |
| Injection Volume | 20 µL |
| Samples | |
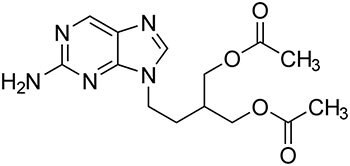
| Standard Solution | 1 μg/mL of USP Famciclovir RS in Mobile Phase A (Figure 1) |
| SST Solution | 0.5 mg/mL of USP Famciclovir System Suitability Mixture RS in Mobile Phase A (Figure 2) |
| Test Solution | Nominally 1 mg/mL of Famciclovir in Mobile Phase A prepared as follows. Transfer an amount equivalent to 250 mg of Famciclovir, from not less than (NLT) 10 finely powdered tablets, to a 250-mL volumetric flask. Add about 125 mL of mobile phase A and sonicate for 30 min with intermittent shaking. Dilute with mobile phase A to volume. (Figure 3) |
| Other samples in monograph method (not shown here) | |
| Peak ID Solution | 4 μg/mL of USP Famciclovir Related Compound A RS and 10 μg/mL of USP Famciclovir Related Compound B RS in Mobile Phase A |
The method acceptance criteria are defined by the relative retention times for Famciclovir related compound A, Famciclovir related compound B, Famciclovir, 6-Chloro famciclovir, and Propionyl famciclovir and are about 0.2, 0.5, 1.0, 1.32, and 1.35, respectively. This application note illustrates with required analytical data that the method meets USP41-NF36 guidelines.
| Gradient | ||
|---|---|---|
| Time | A (%) | B (%) |
| 0 | 95 | 5 |
| 50 | 75 | 25 |
| 60 | 75 | 25 |
| 65 | 95 | 5 |
| 75 | 95 | 5 |
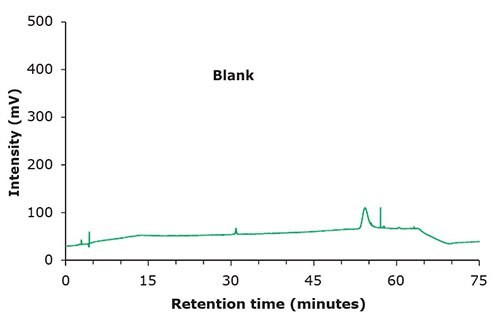
Figure 1. Chromatographic data – blank & standard solution.
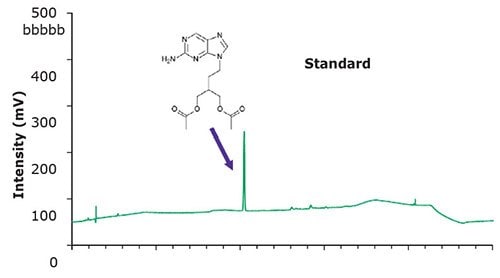
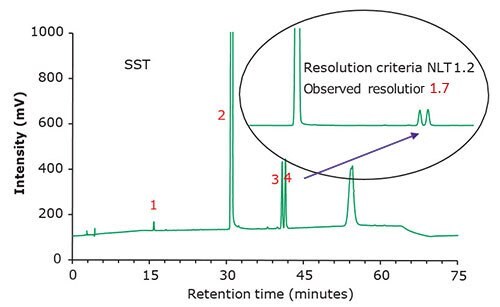
Figure 2.Chromatographic data - system suitability test (SST) solution.
| Peaks | Compound | Retention Time (min) | RRT | Resolution | Tailing Factor |
|---|---|---|---|---|---|
| 1 | Famciclovir Related compound B | 15.8 | 0.51 | - | 0.99 |
| 2 | Famciclovir | 30.8 | 1.00 | 47.9 | 1.02 |
| 3 | 6-Chloro famciclovir | 40.7 | 1.32 | 25.7 | 0.98 |
| 4 | Propionyl famciclovir | 41.4 | 1.34 | 1.7 | 0.98 |
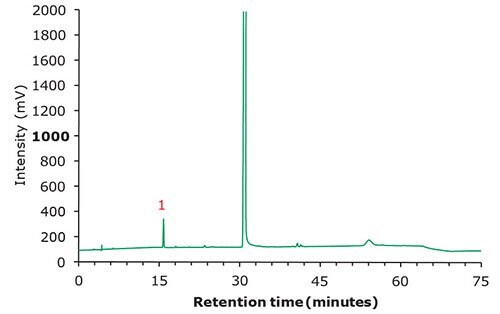
Figure 3. Chromatographic data - test solution.
| Peaks | Compound | Retention Time (min) | RRT | Resolution | Tailing Factor |
|---|---|---|---|---|---|
| 1 | Famciclovir Related compound B | 15.8 | 0.51 | 0 | 0.99 |
| 2 | Famciclovir | 30.8 | 1.00 | 47.5 | 1.02 |
| 3 | 6-Chloro famciclovir | 40.7 | 1.32 | 8.9 | 0.98 |
| 4 | Propionyl famciclovir | 41.4 | 1.34 | 7.7 | 0.98 |
| Peaks | Compound | RT (min) |
|---|---|---|
| 1 | Famciclovir Related compound B | 15.8 |
| 2 | Famciclovir | 30.9 |
| 3 | 6-Chloro famciclovir | 40.7 |
| 4 | Propionyl famciclovir | 41.3 |
2. Standard Repeatability (1 ppm)
| Sample | Area Units |
|---|---|
| STD 1 | 214,771 |
| STD 2 | 213,539 |
| STD 3 | 214,102 |
| STD 4 | 214,935 |
| STD 5 | 214,216 |
| Mean | 214,313 |
| Standard Deviation | 559 |
| RSD (%) | 0.3 |
3. Linearity, LOD & LOQ
| Famciclovir Concentration (μg/mL) | Area Units |
|---|---|
| 0.5 | 59,951 |
| 1 | 98,532 |
| 5 | 478,367 |
| 12.5 | 1,190,770 |
| 25 | 2,360,140 |
| 40 | 3,757,032 |
| 50 | 4,687,957 |
| 60 | 5,618,958 |
| 75 | 7,007,616 |
| LOD (ppm) | 0.3 |
| LOQ (ppm) | 0.9 |
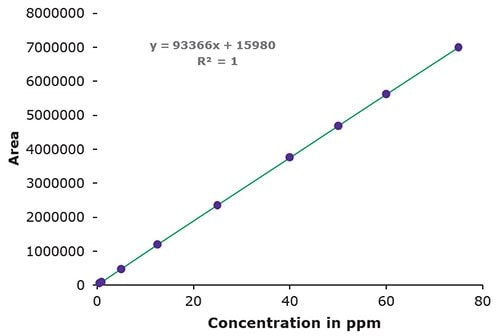
Figure 4.Area / Concentration in ppm
To continue reading please sign in or create an account.
Don't Have An Account?