추천 제품
Quality Level
분석
97%
양식
solid
mp
183-186 °C (subl.) (lit.)
작용기
fluoro
ketone
SMILES string
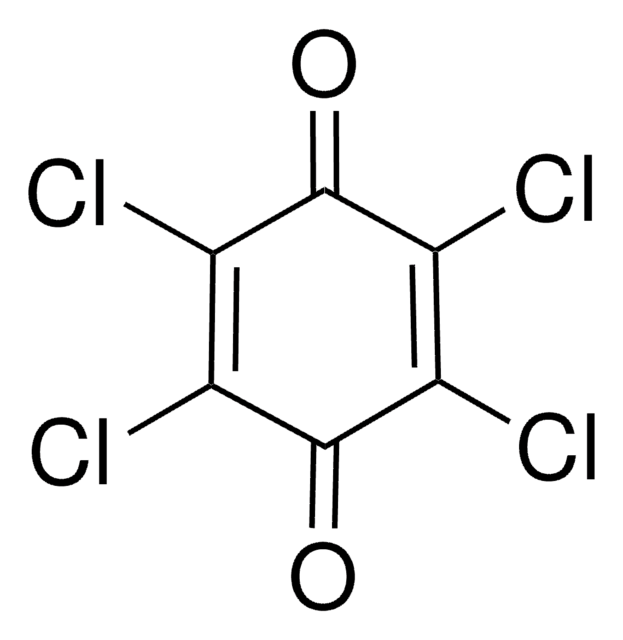
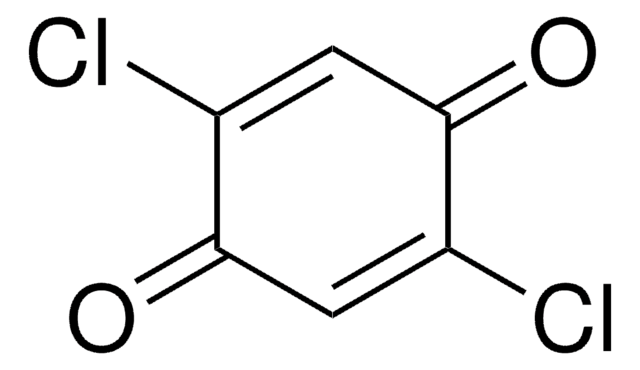
FC1=C(F)C(=O)C(F)=C(F)C1=O
InChI
1S/C6F4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI key
JKLYZOGJWVAIQS-UHFFFAOYSA-N
일반 설명
Tetrafluoro-1,4-benzoquinone is a fluorinated building block, commonly used as a precursor for fluoro derivatives.
애플리케이션
Tetrafluoro-1,4-benzoquinone (fluoranil) can be used to prepare:
- Symmetrical or unsymmetrical ethers by coupling of two alcohols via the oxidation-reduction condensation reaction.
- Azocino[4,3-b]indole scaffold, which is used as an inetermediate to prepare (±)-dasycarpidone.
- Chiral and racemic charge-transfer (CT) complexes with binaphthol.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Ken Okamoto et al.
Journal of the American Chemical Society, 125(41), 12416-12417 (2003-10-09)
Self-promoted electron transfer from a cobalt(II) porphyrin [Co(II)OEP] to p-fluoranil (F4Q) occurs, exhibiting a second-order dependence of the electron-transfer rate with respect to the F4Q concentration due to the formation of a strong complex between the dimer radical anion [(F4Q)2*-]
Generation and spectroscopic characterization of the 2, 3, 5, 6-tetramethoxy-1, 4-benzosemiquinone reactive intermediate.
Mattar SM, et al.
Chemical Physics Letters, 352(1), 39-47 (2002)
Ben-Zhan Zhu et al.
Proceedings of the National Academy of Sciences of the United States of America, 104(45), 17575-17578 (2007-10-31)
We have shown previously that hydroxyl radicals (HO*) can be produced by H2O2 and halogenated quinones, independent of transition metal ions; however, the underlying molecular mechanism is still unclear. In the present study, using the electron spin resonance secondary radical
Tetrafluoro-p-benzoquinone
Essers M and Haufe G
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Complexation Behavior of Binaphthol/Tetrafluoro-1, 4-benzoquinone Charge-Transfer Complex.
Imai Y, et al.
Crystal Growth & Design, 9(5), 2393-2397 (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.