모든 사진(2)
About This Item
Linear Formula:
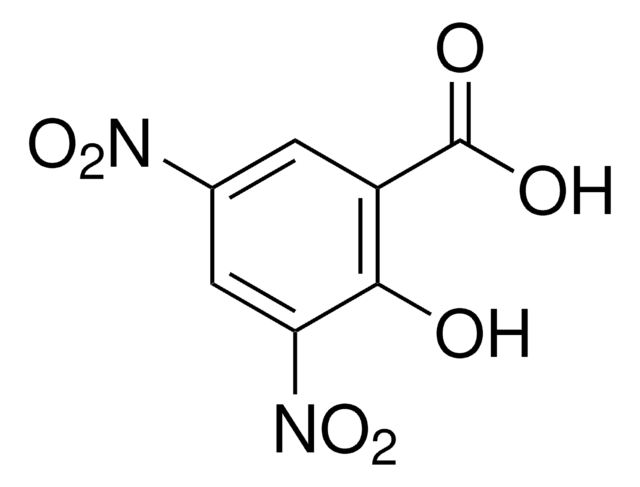
(CH3)2NC10H6SO2NH2
CAS Number:
Molecular Weight:
250.32
Beilstein:
2217203
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
mp
218-221 °C (lit.)
형광
λex 280 nm; λem 470 nm (bound to carbon anhydrase)
λem 580 in ethanol
SMILES string
CN(C)c1cccc2c(cccc12)S(N)(=O)=O
InChI
1S/C12H14N2O2S/c1-14(2)11-7-3-6-10-9(11)5-4-8-12(10)17(13,15)16/h3-8H,1-2H3,(H2,13,15,16)
InChI key
TYNBFJJKZPTRKS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5-(Dimethylamino)-1-naphthalenesulfonamide (DNSA) was used as starting reagent in the synthesis of 2,6-disubstituted pyridines, 6-substituted 2,2′-bipyridines and 6,6′-disubstituted 2,2′-bipyridines. It was also used as fluorescent probe in the determination of concentration of human carbonic anhydrase II-DNSA in solutions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
A Jain et al.
Journal of medicinal chemistry, 37(13), 2100-2105 (1994-06-24)
This paper describes inhibitors for human carbonic anhydrase II (HCAII, EC 4.2.1.1) that bind with nanomolar dissociation constants. These inhibitors were developed by exploiting interactions with hydrophobic "patches" in the lip of the active site of this enzyme. These patches
Raymond Ziessel et al.
Organic letters, 5(14), 2397-2400 (2003-07-05)
[structure: see text] 2,6-Disubstituted pyridines, 6-substituted 2,2'-bipyridines, and 6,6'-disubstituted 2,2'-bipyridines are readily prepared under mild conditions from 5-(dimethylamino)-1-naphthalenesulfonamide chloride (DANS-Cl) and chloromethyl-nitronyl nitroxide (CH(2)Cl-NIT) starting materials and adequately functionalized building blocks. The syntheses of the pyridine molecules bearing two radicals
Jiangxiao Sun et al.
Analytical chemistry, 79(2), 416-425 (2007-01-16)
The interaction between the bovine pancrease trypsin (Tryp) and its competitive inhibitor benzamidine (1), in solution and the gas phase, is investigated using nanoflow electrospray ionization (nanoES) and Fourier transform ion cyclotron resonance mass spectrometry. In a recent study (Clark
Abir L Banerjee et al.
Biochemistry, 44(9), 3211-3224 (2005-03-02)
Benzenesulfonamide and iminodiacetate (IDA)-conjugated Cu(2+) independently interact at the active site and a peripheral site of carbonic anhydrases, respectively [Banerjee, A. L., Swanson, M., Roy, B. C., Jia, X., Haldar, M. K., Mallik, S., and Srivastava, D. K. (2004) J.
Ereny F Morcos et al.
Electrophoresis, 31(22), 3691-3695 (2010-10-26)
Back-scattering interferometry (BSI) is a label-free, free-solution, small-volume technique used for characterizing binding interactions, which is also relevant to a growing number of biosensing applications including drug discovery. Here, we use BSI to characterize the interaction of carbonic anhydrase enzyme
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.