907294
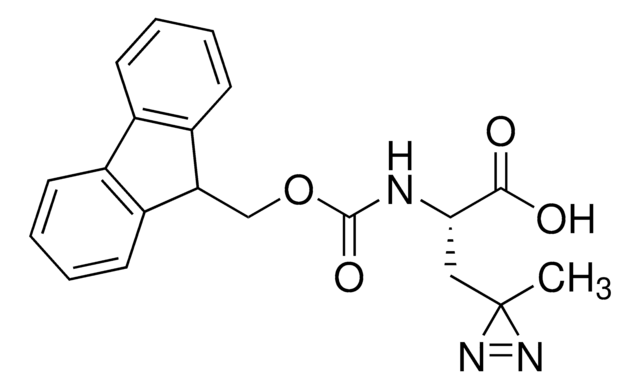
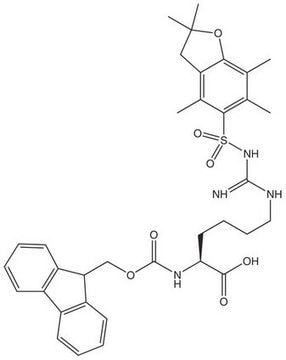
Fmoc-L-Photo-Phe-OH
≥95%
동의어(들):
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C26H20F3N3O4
CAS Number:
Molecular Weight:
495.45
MDL number:
UNSPSC 코드:
12352209
NACRES:
NA.22
추천 제품
분석
≥95%
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
응용 분야
peptide synthesis
작용기
Fmoc
저장 온도
−20°C
애플리케이션
Fmoc-L-Photo-Phe-OH is a diazirine-containing, Fmoc-protected phenylalanine amino acid and multifunctional photo-crosslinker. Its incorporation into peptides or small-molecule probes and tools allows for photoaffinity labeling of cellular targets and protein-protein interactions upon UV light (∼360 nm) irradiation to form a covalent bond. This and other multifunctional probe building blocks will continue to accelerate drug discovery research for probing cellular mechanisms, target ID/validation, and understanding traditionally undruggable targets. An unprotected version is also available as 907340.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
기타 정보
Covalent modifier-type aggregation inhibitor of amyloid-β based on a cyclo-KLVFF motif
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Michiel A Leeuwenburgh et al.
Organic letters, 8(8), 1705-1708 (2006-04-07)
[reaction: see text] A novel solid-phase synthesis strategy toward succinylhydroxamate peptides, using an appropriately protected hydroxamate building block, is described. Rapid and efficient access is gained to amine-functionalized peptides, which can be decorated with, for instance, a fluorescent label. In
Ryuto Kino et al.
Bioorganic & medicinal chemistry letters, 25(15), 2972-2975 (2015-06-06)
Inhibition of amyloid-β (Aβ) aggregation could be a drug development target for treating Alzheimer disease. Insufficient activity to inhibit aggregation, however, remains a key issue. Here, we report a covalent modifier-type aggregation inhibitor of Aβ, diazirine-equipped cyclo-KLVF(β-Ph)F (2). Due to
Martijn W H Pinkse et al.
The Journal of biological chemistry, 284(24), 16354-16368 (2009-04-17)
The inhibitor peptide DT-2 (YGRKKRRQRRRPPLRKKKKKH) is the most potent and selective inhibitor of the cGMP-dependent protein kinase (PKG) known today. DT-2 is a construct of a PKG tight binding sequence (W45, LRKKKKKH, KI=0.8 microM) and a membrane translocating sequence (DT-6
Dany Fillion et al.
Journal of medicinal chemistry, 49(7), 2200-2209 (2006-03-31)
A stereospecific convergent synthesis of N-[(9-fluorenyl)methoxycarbonyl]-p-[3-(trifluoromethyl)-3H-diazirin-3-yl]-l-phenylalanine (Fmoc-12, Fmoc-Tdf) and its incorporation into the C-terminal position of the angiotensin II (AngII) peptide to form (125)I[Sar(1),Tdf(8)]AngII ((125)I-13) is presented. This amino acid photoprobe is a highly reactive carbene-generating diazirine phenylalanine derivative that
R Falchetto et al.
The Journal of biological chemistry, 266(5), 2930-2936 (1991-02-15)
A synthetic, 28-residue peptide derived from the calmodulin-binding sequence of the plasma membrane Ca2+ pump (C28W) inhibits the ATPase activity of a calpain-produced, truncated fragment of the enzyme. The fragment, which has lost the calmodulin-binding domain, has a molecular mass
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.