추천 제품
분석
95%
형태
(Liquid or Semi-Solid or Paste or Solid)
반응 적합성
reaction type: click chemistry
reagent type: cross-linking reagent
작용기
azide
carboxylic acid
저장 온도
2-8°C
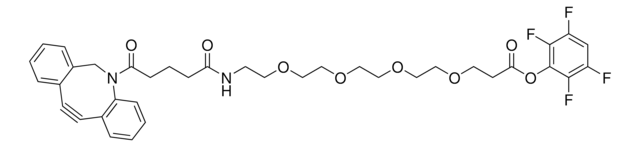
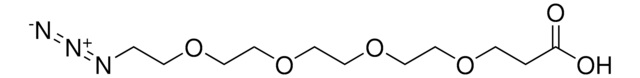
SMILES string
OC(COCCOCCOCCOCCOCCOCCN=[N+]=[N-])=O
애플리케이션
This heterobifunctional, PEGylated crosslinker 20-Azido-3,6,9,12,15,18-hexaoxaicosanoic acid features a carboxyl group at one end and azide at the other. The hydrophillic PEG linker facilitates solubility in biological applications. This azido-PEG6-acid linker can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
법적 정보
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
관련 제품
제품 번호
설명
가격
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Yong Ma et al.
Bioconjugate chemistry, 21(11), 1994-1999 (2010-10-14)
A surface-bound cytomimetic assembly based on chemically selective and biocompatible immobilization and further modification of intact liposome is described. Liposomes carrying PEG-triphenylphosphine were chemoselectively immobilized onto azide-modified glass slides through Staudinger ligation, followed by modification with azide-modified lactose as a
Towards potential nanoparticle contrast agents: Synthesis of new functionalized PEG bisphosphonates.
Souad Kachbi-Khelfallah et al.
Beilstein journal of organic chemistry, 12, 1366-1371 (2016-08-26)
The use of nanotechnologies for biomedical applications took a real development during these last years. To allow an effective targeting for biomedical imaging applications, the adsorption of plasmatic proteins on the surface of nanoparticles must be prevented to reduce the
Joel Hwang et al.
Chemical communications (Cambridge, England), 50(24), 3159-3162 (2014-01-30)
Oligo(ethylene glycol)-linked light fluorous tags have been found to be optimal for conjugating to glycans for both high-yield enzymatic glycosylation reactions using one-pot multienzyme (OPME) systems and quick product purification using fluorous solid-phase extraction (FSPE) cartridges. The combination of OPME
Ryota Sato et al.
Journal of the American Chemical Society, 139(48), 17397-17404 (2017-11-10)
Single-molecule imaging (SMI) has been widely utilized to investigate biomolecular dynamics and protein-protein interactions in living cells. However, multicolor SMI of intracellular proteins is challenging because of high background signals and other limitations of current fluorescence labeling approaches. To achieve
Mingcheng Qian et al.
ChemMedChem, 13(9), 944-956 (2018-02-17)
Currently, there is mounting evidence that intermolecular receptor-receptor interactions may result in altered receptor recognition, pharmacology and signaling. Heterobivalent ligands have been proven useful as molecular probes for confirming and targeting heteromeric receptors. This report describes the design and synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.