모든 사진(1)
About This Item
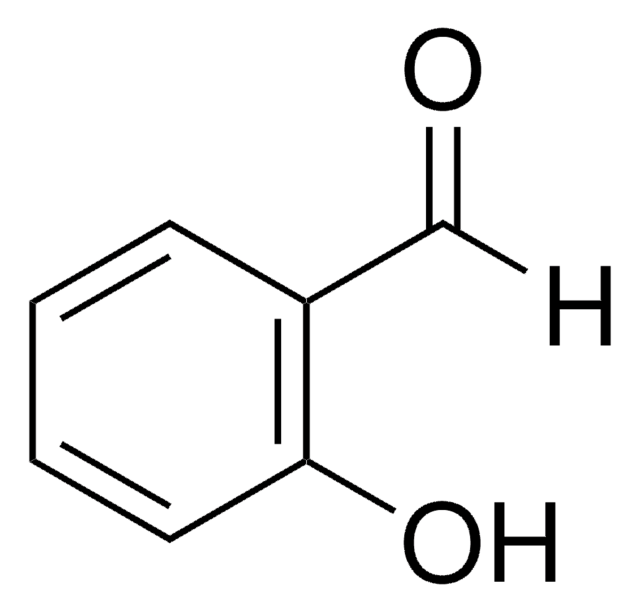
Linear Formula:
HOC6H4CHO
CAS Number:
Molecular Weight:
122.12
FEMA Number:
3984
Beilstein:
471352
EC Number:
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
5.047
NACRES:
NA.21
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
규정 준수
EU Regulation 1223/2009
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 110
분석
≥97%
mp
112-116 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
향수 알레르기항원
no known allergens
감각 수용성의
almond; balsamic; nutty; sweet
SMILES string
[H]C(=O)c1ccc(O)cc1
InChI
1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
InChI key
RGHHSNMVTDWUBI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-hydroxybenzaldehyde occurs naturally in vanilla beans and is one of the keys contributors to the vanilla flavor.
애플리케이션
- Dual action of benzaldehydes: Inhibiting quorum sensing and enhancing antibiotic efficacy for controlling Pseudomonas aeruginosa biofilms.: This paper highlights the dual functionality of benzaldehyde derivatives in inhibiting bacterial communication and boosting antibiotic efficiency, offering promising strategies against biofilm-associated infections (Borges A et al., 2024).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
345.2 °F
Flash Point (°C)
174 °C
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Unusual 4-hydroxybenzaldehyde synthase activity from tissue cultures of the vanilla orchid Vanilla planifolia.
Podstolski A, et al.
Phytochemistry, 61(6), 611-620 (2002)
Huan Wen et al.
European journal of medicinal chemistry, 43(1), 166-173 (2007-06-19)
A series of helicid analogues were prepared and evaluated in vitro for the cholinesterase (AChE and BuChE) inhibitory activities via UV spectroscopy. The results indicated that compounds 5, 6d and 8 exhibited potent AChE inhibitory activities with IC(50) values of
Nadja Schultz-Jensen et al.
Applied biochemistry and biotechnology, 165(3-4), 1010-1023 (2011-07-06)
The potential of wheat straw for ethanol production after pretreatment with O(3) generated in a plasma at atmospheric pressure and room temperature followed by fermentation was investigated. We found that cellulose and hemicellulose remained unaltered after ozonisation and a subsequent
Silvia Forcat et al.
Phytochemistry, 71(8-9), 870-876 (2010-04-03)
Changes occurring to plant cell walls were examined following inoculation of Arabidopsis leaves with pathogenic and non-pathogenic (hrpA mutant) strains of Pseudomonas syringae pv. tomato. We have targeted low molecular weight, cross-linked phenolic and indolic compounds that were released from
Qin Yan et al.
European journal of medicinal chemistry, 44(10), 4235-4243 (2009-06-26)
A series of novel 5-benzylidene barbiturate and thiobarbiturate derivatives were synthesized and evaluated as tyrosinase inhibitors and antibacterial agents. The results demonstrated that some compounds had more potent inhibitory activities than the parent compound 4-hydroxybenzaldehyde (IC(50)=1.22 mM). Particularly, compounds 1a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.