추천 제품
형태
powder
포장
pkg of 1 × 10 mg (860602P-10mg)
pkg of 1 × 5 mg (860602P-5mg)
제조업체/상표
Avanti Research™ - A Croda Brand 860602P
지질 유형
sphingolipids
배송 상태
dry ice
저장 온도
−20°C
SMILES string
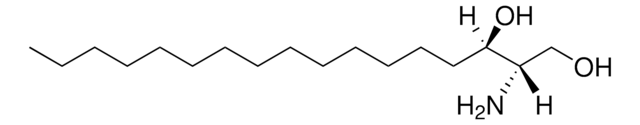
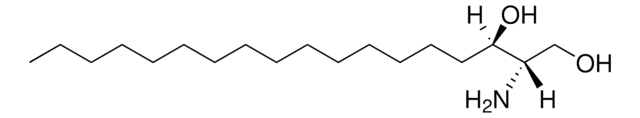
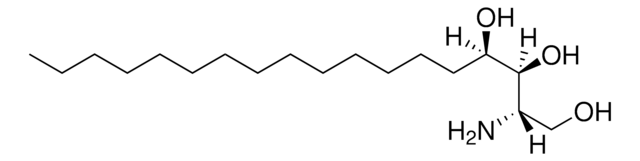
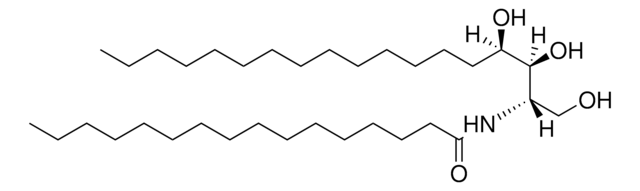
O[C@]([H])(CCCCCCCCCCCCC)[C@](O)([H])[C@](N)([H])CO
일반 설명
D-ribo-phytosphingosine (C17 base), also known as 4D-hydroxysphinganine or PHS, is widely found in membranes of fungi, plants, bacteria, marine organisms and mammalian tissues.
애플리케이션
D-ribo-phytosphingosine (C17 base) has been used as a standard for the quantification of total plant long-chain bases (LCB) by gas chromatography-mass spectrometry (GC-MS).
생화학적/생리학적 작용
D-ribo-phytosphingosine helps in maintaining the structural integrity of membrane. It also controls cellular growth and mediates the heat stress response of yeast. In addition, PHS acts as a precursor for synthesis of various key lipid mediators including PHS 1-phosphate, inositol phosphorylceramide and KRN7000 (α-anomer of galactosylceramide). This phospholipid also has an ability to stimulate keratinocyte differentiation. Therefore, PHS is used as an active constituent in cosmetic formulations.
포장
5 mL Amber Glass Screw Cap Vial (860602P-10mg)
5 mL Amber Glass Screw Cap Vial (860602P-5mg)
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Polar emollients in cosmetic formulations enhance the penetration and biological effects of Phytosphingosine on skin
Schiemann Y, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 331(1-2), 103-107 (2008)
Asymmetric synthesis of d-ribo-phytosphingosine from 1-tetradecyne and (4-methoxyphenoxy) acetaldehyde
Liu Z, et al.
The Journal of Organic Chemistry, 75(13), 4356-4364 (2010)
Zheng Liu et al.
The Journal of organic chemistry, 75(13), 4356-4364 (2010-06-10)
An asymmetric synthesis of d-ribo-phytosphingosine (1) was achieved by utilizing the ProPhenol (12)-catalyzed alkynylation of unsaturated aldehyde 8 to afford allylic propargylic alcohol (S)-6 followed by asymmetric epoxidation and opening of propargylic epoxy alcohol anti-5 with NaN(3)/NH(4)Cl. Deprotection and reduction
Jean-Luc Cacas et al.
Analytical and bioanalytical chemistry, 403(9), 2745-2755 (2012-05-12)
In eukaryotic organisms, sphingolipids are major structural lipids of biological membranes and perform additional essential functions as signalling molecules. While long-chain bases (LCB), the common precursor to all sphingolipid classes, is represented by only one major molecular species in animals
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.