추천 제품
product name
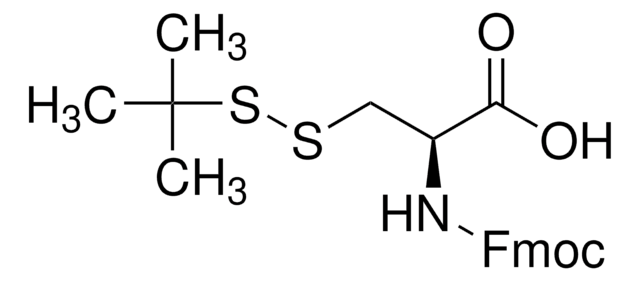
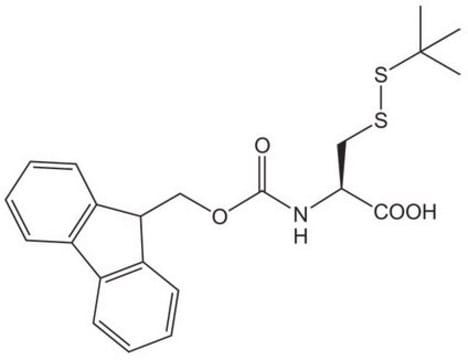
Fmoc-Cys(Mmt)-OH, Novabiochem®
Quality Level
제품 라인
Novabiochem®
분석
≥85.0% (acidimetric)
≥98.0% (HPLC)
≥99% (TLC)
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
응용 분야
peptide synthesis
작용기
thiol
저장 온도
15-25°C
InChI
1S/C38H33NO5S/c1-43-29-22-20-28(21-23-29)38(26-12-4-2-5-13-26,27-14-6-3-7-15-27)45-25-35(36(40)41)39-37(42)44-24-34-32-18-10-8-16-30(32)31-17-9-11-19-33(31)34/h2-23,34-35H,24-25H2,1H3,(H,39,42)(H,40,41)/t35-/m0/s1
InChI key
LOBUWFUSGOYXQX-DHUJRADRSA-N
일반 설명
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Fmoc SPPS of Cysteine-Containing Peptides
Literature references
[1] K. Barlos, et al. in ′Peptides 1992, Proc. 22nd European Peptide Symposium′, C. H. Schneider & A. N. Eberle (Eds), ESCOM, Leiden, 1993, pp. 283.
[2] C. Kellenberger, et al. (1995) Pept. Res., 8, 321.
[3] K. Barlos, et al. (1996) Int. J. Peptide Protein Res., 47, 148.
[4] A. K. Galande, et al. (2005) J. Comb. Chem., 7, 174.
애플리케이션
- An Optimized Scalable Fully Automated Solid-Phase Microwave-Assisted cGMP-Ready Process for the Preparation of Eptifibatide: Details the use of Fmoc-Cys(Mmt)-OH in the synthesis of Eptifibatide, highlighting its role in a cGMP-ready process (Sabatino et al., 2020).
결합
분석 메모
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(011A)): ≥ 99 %
Purity (TLC(0811)): ≥ 99 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Assay (acidimetric): ≥ 85.0 %
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
프로토콜
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.