46648
Praziquantel
VETRANAL®, analytical standard
동의어(들):
2-(Cyclohexylcarbonyl)-1,2,3,6,7-11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H24N2O2
CAS Number:
Molecular Weight:
312.41
Beilstein:
761557
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
제품 라인
VETRANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
항생제 활성 스펙트럼
parasites
응용 분야
clinical testing
형식
neat
동작 모드
cell membrane | interferes
저장 온도
2-8°C
SMILES string
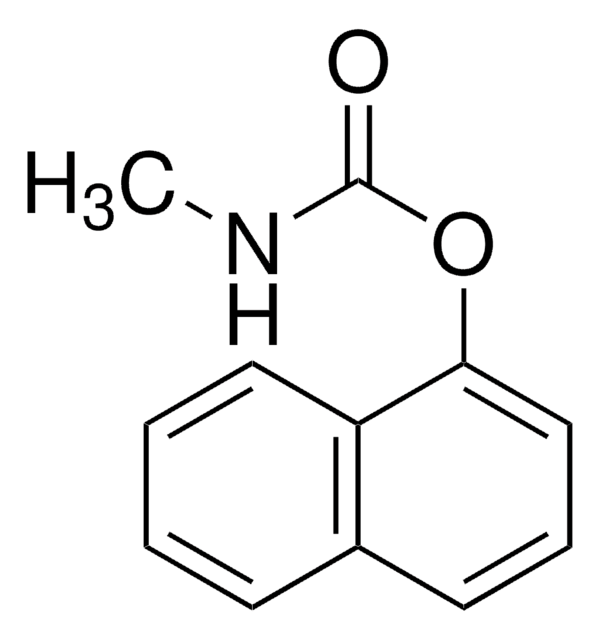
O=C1CN(CC2N1CCc3ccccc23)C(=O)C4CCCCC4
InChI
1S/C19H24N2O2/c22-18-13-20(19(23)15-7-2-1-3-8-15)12-17-16-9-5-4-6-14(16)10-11-21(17)18/h4-6,9,15,17H,1-3,7-8,10-13H2
InChI key
FSVJFNAIGNNGKK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: quinolone
Praziquantel (PZQ) is a pyrazino-isoquinoline compound used as a broad-spectrum anthelmintic drug against trematode and cestode infections.
애플리케이션
PZQ may be used as a reference standard for the determination for PZQ in:
- Surface water samples by solid phase extraction (SPE) and ultra-high performance liquid chromatography-quadrupole linear ion trap tandem mass spectrometry (UHPLC-QqLIT-MS) equipped with electrospray ionization (ESI) source.
- Plants by HPLC with triple quadrupole mass spectrometry (HPLC-ESI-QqQ-MS/MS) operating in multiple reaction monitoring (MRM) mode.
- Tablet formulations by reversed phase (RP) HPLC with ultraviolet (UV) detection.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
법적 정보
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Study of praziquantel phytoremediation and transformation and its removal in constructed wetland.
Marsik P, et al.
Journal of Hazardous Materials, 323, 394-399 (2017)
Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry.
Zrncic M, et al.
Chemosphere, 99, 224-232 (2014)
A rapid stability indicating LC-method for determination of praziquantel in presence of its pharmacopoeial impurities.
Hashem H, et al.
Arabian Journal of Chemistry, 10, S35-S41 (2017)
Development and validation of a dissolution test method for albendazole and praziquantel in their combined dosage form.
Vignaduzzo S, et al.
Journal of the Brazilian Chemical Society, 26(4), 729-735 (2015)
Development and validation of an enantioselective LC-MS/MS method for the analysis of the anthelmintic drug praziquantel and its main metabolite in human plasma, blood and dried blood spots.
Meister I, et al.
Journal of Pharmaceutical and Biomedical Analysis, 118, 81-88 (2016)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.