PHR1500
Imidurea
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
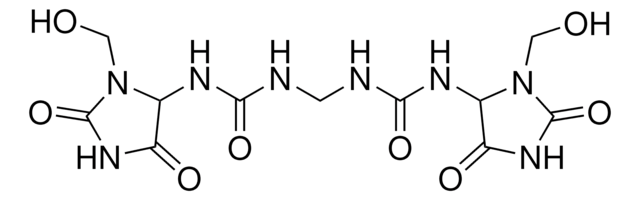
Imidazolidinyl urea, N,N′′-Methylenebis[N′-[3-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl]-urea
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C11H16N8O8
CAS Number:
Molecular Weight:
388.29
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to USP 1336806
API family
imidurea
CofA
current certificate can be downloaded
포장
pkg of 1 g
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
OCN1C(NC(=O)NCNC(=O)NC2N(CO)C(=O)NC2=O)C(=O)NC1=O
InChI
1S/C11H16N8O8/c20-2-18-4(6(22)16-10(18)26)14-8(24)12-1-13-9(25)15-5-7(23)17-11(27)19(5)3-21/h4-5,20-21H,1-3H2,(H2,12,14,24)(H2,13,15,25)(H,16,22,26)(H,17,23,27)
InChI key
ZCTXEAQXZGPWFG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Imidurea may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Used in:
Pharmacological studies investigating nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis
Therapeutic studies involving nanostructured vegetable-based carriers for topical delivery of active molecules
Topical formulas for use as antioxidants
Pharmacological studies investigating nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis
Therapeutic studies involving nanostructured vegetable-based carriers for topical delivery of active molecules
Topical formulas for use as antioxidants
분석 메모
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAA3048 in the slot below. This is an example certificate only and may not be the lot that you receive.
Validation of a micellar electrokinetic capillary chromatography method for the determination of imidurea, methyl and propylparabens in a pharmaceutical ointment
Baalbaki B, et al.
Analytica Chimica Acta, 463(1), 15-20 (2002)
Analysis of preservative in pharmaceutical products
Fahelelbom KMS, et al.
Pharmaceutical Reviews, 5(1), 3960-3968 (2007)
María Castro-Puyana et al.
Electrophoresis, 26(20), 3960-3968 (2005-10-12)
EKC using a neutral CD as chiral selector was applied in this work to the development of a method enabling the enantiomeric separation of ketoconazole and terconazole antifungals. The influence of different experimental conditions such as temperature, CD concentration, pH
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.