Y0000550
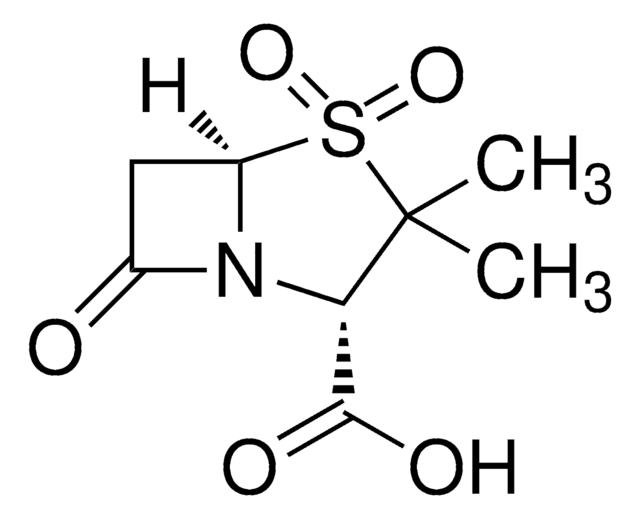
Sulbactam for peak identification
European Pharmacopoeia (EP) Reference Standard
동의어(들):
Sulbactam, (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide, Betamaze, CP 45899, CP-45,899, Penicillanic acid 1,1-dioxide, Penicillanic acid S,S-dioxide, Penicillanic acid dioxide, Penicillanic acid sulfone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C8H11NO5S
CAS Number:
Molecular Weight:
233.24
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
sulbactam
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
CC1(C)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(O)=O
InChI
1S/C8H11NO5S/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14/h5-6H,3H2,1-2H3,(H,11,12)/t5-,6+/m1/s1
InChI key
FKENQMMABCRJMK-RITPCOANSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Sulbactam for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
G S Singh
Mini reviews in medicinal chemistry, 4(1), 93-109 (2004-02-03)
Beta-lactam ring-containing compounds such as penicillins, ampicillin, amoxicillin, cephalosporins and carbapenems are among the most famous antibiotics. This article reviews the recent developments in study of cephems, oxacephems, penams and sulbactam. Many of the compounds reviewed have potential antibacterial activity
A S Levin
Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 8(3), 144-153 (2002-05-16)
Recent studies have highlighted the emergence of infections involving multiresistant Acinetobacter clinical isolates. Sulbactam offers direct antimicrobial activity against Acinetobacter species. Accordingly, co-administration of sulbactam with ampicillin or cefoperazone offers the potential of effective empirical therapy against Acinetobacter and other
Arbab Khan et al.
PloS one, 9(9), e108246-e108246 (2014-09-30)
The use of three classical β-lactamase inhibitors (Clavulanic acid, tazobactam and sulbactam) in combination with β-lactam antibiotics is presently the mainstay of antibiotic therapy against Gram-negative bacterial infections. However these inhibitors are unable to inhibit carbapenemase KPC-2 effectively. They being
J D Williams
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 24(3), 494-497 (1997-03-01)
beta-Lactamase inhibitors such as sulbactam are beta-lactam compounds that have low antimicrobial activity but are able to inhibit enzymes (beta-lactamases) that destroy beta-lactam antibiotics like penicillins and cephalosporins. The main activity of beta-lactamase inhibitors is directed against plasmid-mediated transferable enzymes
M Akova
Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 14 Suppl 1, 185-188 (2007-12-25)
Sulbactam irreversibly inhibits the hydrolytic activity of beta-lactamases. This compound is commercially available in combination with either ampicillin or cefoperazone. In each instance, the activity of the partner antibiotic against beta-lactamase-producing bacteria is restored. One of the particular advantages of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.