추천 제품
생물학적 소스
synthetic (organic)
분석
≥98%
형태
powder
불순물
inosine, essentially free
solubility
1 M NH4OH: 50 mg/mL, clear, colorless
저장 온도
−20°C
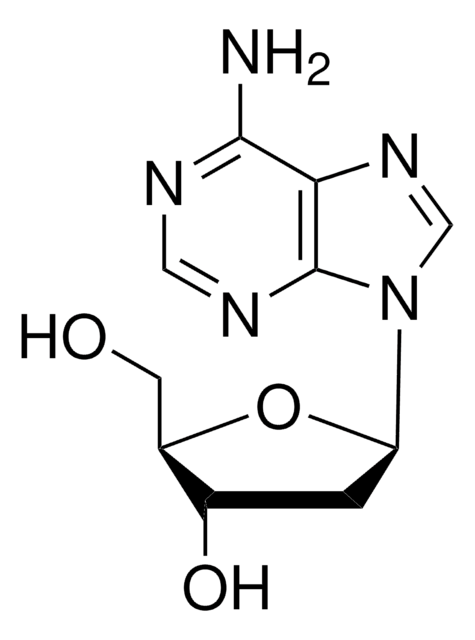
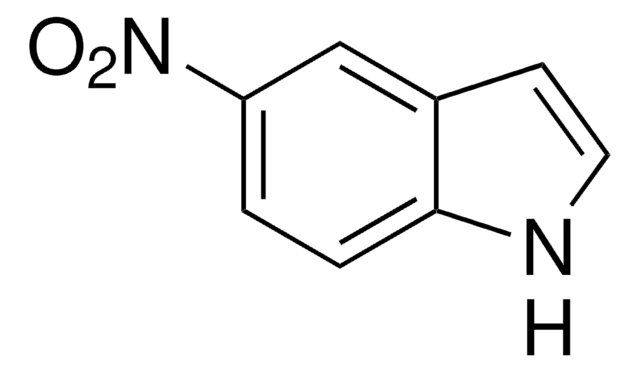
SMILES string
OC[C@H]1O[C@H](C[C@@H]1O)n2cnc3C(=O)NC=Nc23
InChI
1S/C10H12N4O4/c15-2-6-5(16)1-7(18-6)14-4-13-8-9(14)11-3-12-10(8)17/h3-7,15-16H,1-2H2,(H,11,12,17)/t5-,6+,7+/m0/s1
InChI key
VGONTNSXDCQUGY-RRKCRQDMSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
2′-Deoxyinosine has been used in the quantification of nucleoside forms of DNA lesion in a single DNA sample by liquid chromatography tandem mass spectrometry (LC-MS/MS). It has also been used as a standard for high performance liquid chromatography analysis.
생화학적/생리학적 작용
2′-Deoxyinosine is a nucleoside composed of hypoxanthine attached to 2′-deoxyribose via a β-N9-glycosidic bond. 2′-Deoxyinosine in DNA can arise from deamination of adenosine. 2′-deoxyinsine can be used as a model compound to study the chemistry of adduct formation and radical chemistry that may affect DNA structures. 2′-Deoxyinosine is used to produce hybridization-sensitive fluorescent DNA probes with self-avoidance ability.
2′-Deoxyinosine is a nucleoside form of hypoxanthine. It is a DNA damage product resulting from the impairment of DNA by reactive nitrogen species. 2′-deoxyinosine is formed from nitrosative deamination by N2O3.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Shuji Ikeda et al.
Organic & biomolecular chemistry, 8(3), 546-551 (2010-01-22)
Hybridization-sensitive fluorescent probes have an inherent disadvantage: self-dimerization of the probe prevents the fluorescence quenching prior to hybridization with the target, resulting in a high background signal. To avoid self-dimerization of probes, we focused on a base pair formed by
Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation.
Bo Pang et al.
Carcinogenesis, 28(8), 1807-1813 (2007-03-10)
In an effort to define the prevalent DNA damage chemistry-associated chronic inflammation, we have quantified 12 DNA damage products in tissues from the SJL mouse model of nitric oxide (NO) overproduction. Using liquid chromatography-mass spectrometry/MS and immunoblot techniques, we analyzed
Quantification of DNA damage products resulting from deamination, oxidation and reaction with products of lipid peroxidation by liquid chromatography isotope dilution tandem mass spectrometry
Taghizadeh K, et al.
Nature Protocols, 3(8), 1287-1287 (2008)
Bernard Weiss
DNA repair, 7(2), 205-212 (2007-11-06)
Deoxyinosine (dI) is produced in DNA by the hydrolytic or nitrosative deamination of deoxyadenosine. It is excised in a repair pathway that is initiated by endonuclease V, the product of the nfi gene. The repair was studied in vivo using
Manabu Yasui et al.
Journal of molecular biology, 377(4), 1015-1023 (2008-02-29)
Chronic inflammation involving constant generation of nitric oxide (*NO) by macrophages has been recognized as a factor related to carcinogenesis. At the site of inflammation, nitrosatively deaminated DNA adducts such as 2'-deoxyinosine (dI) and 2'-deoxyxanthosine are primarily formed by *NO
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.