1269447
USP
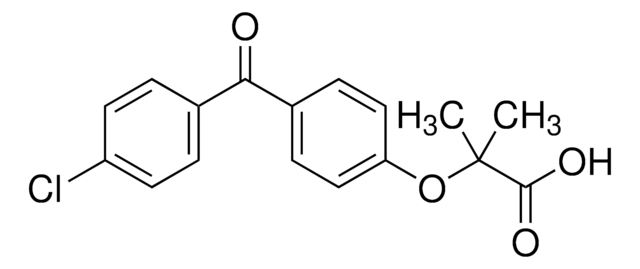
Fenofibrate
United States Pharmacopeia (USP) Reference Standard
동의어(들):
2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid isopropyl ester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H21ClO4
CAS Number:
Molecular Weight:
360.83
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
fenofibrate
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
CC(C)OC(=O)C(C)(C)Oc1ccc(cc1)C(=O)c2ccc(Cl)cc2
InChI
1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3
InChI key
YMTINGFKWWXKFG-UHFFFAOYSA-N
유전자 정보
human ... PPARA(5465)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Fenofibrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Fenofibrate Capsules
- Fenofibrate Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
STOT RE 2 Oral
표적 기관
Liver
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Samuel Rommelaere et al.
PloS one, 9(8), e104925-e104925 (2014-08-21)
Liver is a major regulator of lipid metabolism and adaptation to fasting, a process involving PPARalpha activation. We recently showed that the Vnn1 gene is a PPARalpha target gene in liver and that release of the Vanin-1 pantetheinase in serum
Pascale Massin et al.
Ophthalmic epidemiology, 21(5), 307-317 (2014-08-19)
Fenofibrate reduced progression of diabetic retinopathy in two large randomized studies. The effect of 135 mg fenofibric acid on diabetic macular edema (DME) was evaluated in subjects with existing DME. In this double-blind, randomized, placebo-controlled study, 110 subjects with DME not
Michel P Hermans
Diabetes & vascular disease research, 8(3), 180-189 (2011-05-18)
Microvascular complications are common in type 2 diabetes in primary care. Intensified management of glycaemia or blood pressure had little effect on microvascular complication rates in recent large trials (ADVANCE, VADT, ACCORD). In 2005, the FIELD study demonstrated a significant
Kate McKeage et al.
Drugs, 71(14), 1917-1946 (2011-09-29)
Fenofibrate is a fibric acid derivative indicated for the treatment of severe hypertriglyceridaemia and mixed dyslipidaemia in patients who have not responded to nonpharmacological therapies. The lipid-modifying effects of fenofibrate are mediated by the activation of peroxisome proliferator-activated receptor-α. Fenofibrate
Pitchai Balakumar et al.
Pharmacological research, 63(1), 8-12 (2010-11-26)
Fenofibrate is a third-generation fibric acid derivative employed clinically as a hypolipidemic agent to lessen the risk caused by atherosclerosis. Dyslipidemia is a condition associated with elevated levels of low-density lipoproteins (LDL), very low-density lipoproteins (VLDL) and triglycerides, and reduced
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.