196282
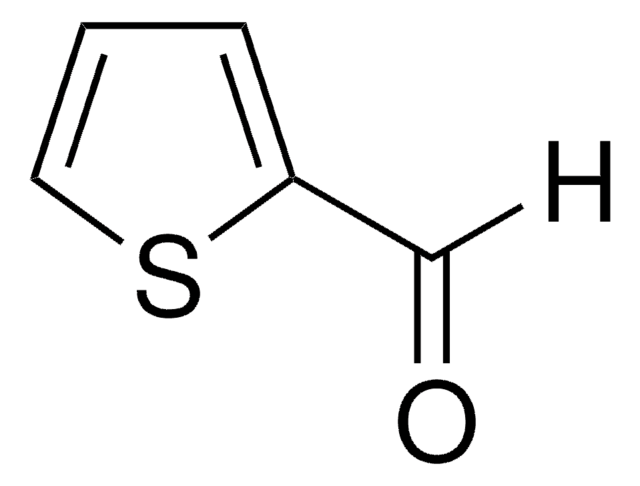
3-Thiophenecarboxaldehyde
98%
Synonym(s):
3-Formylthiophene, 3-Thienaldehyde, 3-Thienylcarboxaldehyde, 3-Thiophenealdehyde, 3-Thiophenecarbaldehyde, Thiofuran-3-carboxaldehyde, Thiophen-3-aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4OS
CAS Number:
Molecular Weight:
112.15
Beilstein/REAXYS Number:
105889
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.31 mmHg ( 20 °C)
Quality Level
assay
98%
form
liquid
autoignition temp.
>392 °F
refractive index
n20/D 1.583 (lit.)
bp
194-196 °C (lit.)
86-87 °C/20 mmHg (lit.)
density
1.28 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccsc1
InChI
1S/C5H4OS/c6-3-5-1-2-7-4-5/h1-4H
InChI key
RBIGKSZIQCTIJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Thiophenecarboxaldehyde has been used in the synthesis of:
- series of 4-substituted 2-thiophenesulfonamides
- acetal and ketal derivatives of 4′-demethylepipodophyllotoxin-β-D-glucoside and epipodophyllotoxin-β-D-glucoside
- 1,2-di-3-thienyl-2-hydroxyethanone (3,3′-thenoin), 3-thienyl symmetric analog of benzoin
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1, 2-Di-3-thienyl-2-hydroxyethanone (3, 3′-thenoin).
Crundwell G, et al.
Acta Crystallographica Section E, Structure Reports Online, 58(6), o668-o670 (2002)
R S Gupta et al.
Anti-cancer drug design, 2(1), 1-12 (1987-08-01)
We have synthesized acetal and ketal derivatives of 4'-demethylepipodophyllotoxin-beta-D-glucoside (DMEPG) and epipodophyllotoxin-beta-D-glucoside (EPG) with a number of different aldehydes (viz. acetaldehyde, propionaldehyde, 2-thiophenecarboxaldehyde, 3-thiophenecarboxaldehyde, 2-furancarboxaldehyde, benzaldehyde, phenylacetaldehyde, hydrocinnamaldehyde) and acetone. The cross resistance of these compounds towards a set of
J M Holmes et al.
Journal of medicinal chemistry, 37(11), 1646-1651 (1994-05-27)
A series of 4-substituted 2-thiophenesulfonamides was prepared from 3-thiophenecarboxaldehyde using metalation chemistry developed for 3-furaldehyde. Several of these compounds inhibit carbonic anhydrase II in vitro at concentrations of less than 10 nM. In addition, none of these compounds exhibit sensitization
Kollur Shiva Prasad et al.
Molecules (Basel, Switzerland), 25(12) (2020-06-26)
Herein we report the synthesis and structural elucidation of two novel imine-based ligands, 2-(1,10-phenanthrolin-5-yl)imino)methyl)-5-bromophenol (PIB) and N-(1,10-phenanthrolin-5-yl)-1-(thiophen-3-yl)methanimine (PTM) ligands. An in vitro cytotoxicity assay of the synthesized molecules was carried out against breast, cervical, colorectal, and prostate cancer cell lines
Kyungsil Yoon et al.
Cells, 8(3) (2019-03-15)
Chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is an orphan receptor and member of the nuclear receptor superfamily. Among a series of methylene substituted diindolylmethanes (C-DIMs) containing substituted phenyl and heteroaromatic groups, we identified 1,1-bis(3'-indolyl)-1-(4-pyridyl)-methane (DIM-C-Pyr-4) as an activator of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service