C112402
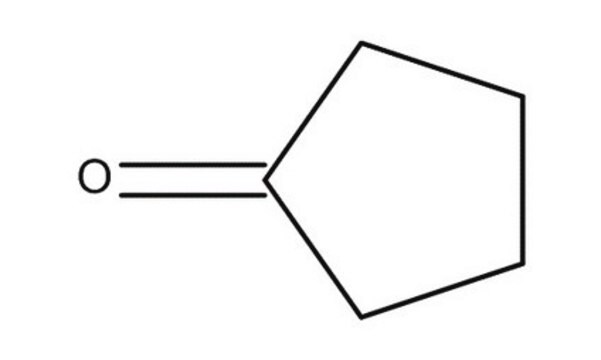
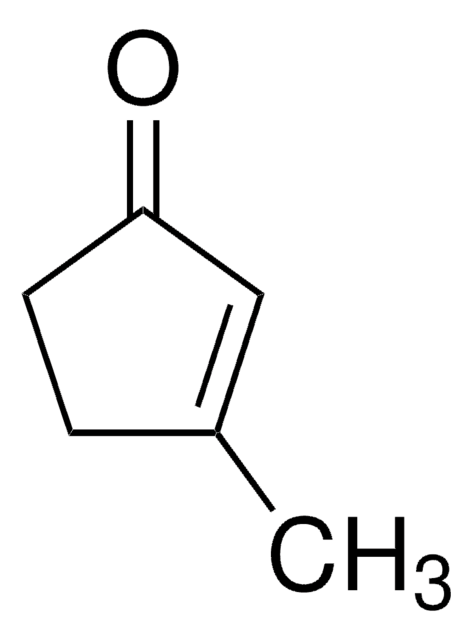
Cyclopentanone
ReagentPlus®, ≥99%
Synonym(s):
Adipic ketone
About This Item
Recommended Products
Quality Level
product line
ReagentPlus®
assay
≥99%
form
liquid
refractive index
n20/D 1.437 (lit.)
bp
130-131 °C (lit.)
mp
−51 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCC1
InChI
1S/C5H8O/c6-5-3-1-2-4-5/h1-4H2
InChI key
BGTOWKSIORTVQH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It may be used in the following studies:
- Preparation of (2E,5E)-2,5-bis(4-(azidomethyl)benzylidene) cyclopentanone, via cross-aldol condensation.
- Preparation of symmetrical C-5 curcuminoids by reacting with substituted benzaldehyde via Claisen-Schmidt condensation.
- As an electron pair donor to stabilize allyl and vinyl cations during intramolecular carbohydroxylation of alkynes.
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
86.0 °F - closed cup
flash_point_c
30 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The aldol condensation reaction is an organic reaction introduced by Charles Wurtz, who first prepared the β-hydroxy aldehyde from acetaldehdye in 1872.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service