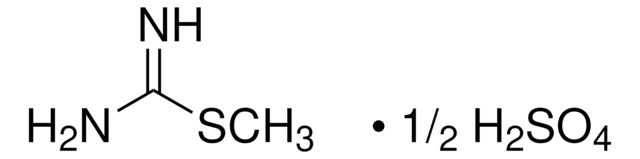All Photos(1)
About This Item
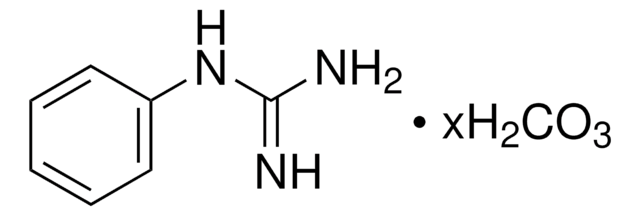
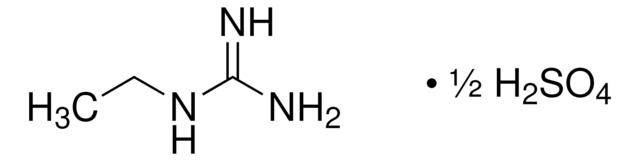
Linear Formula:
CH5N3O · 1/2H2SO4
CAS Number:
Molecular Weight:
124.11
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic (organic)
assay
≥98% (TLC)
form
powder
solubility
water: 25 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
NC(=N)NO.NC(=N)NO.OS(O)(=O)=O
InChI
1S/2CH5N3O.H2O4S/c2*2-1(3)4-5;1-5(2,3)4/h2*5H,(H4,2,3,4);(H2,1,2,3,4)
InChI key
MTGDDPZRXSDPFH-UHFFFAOYSA-N
Biochem/physiol Actions
An early antitumor agent. Oxidation results in release of NO, and formation of other reactive oxygen species, including peroxynitrite and peroxyl radicals. Reacts with NO to form an adduct which is a potent and stable vasodilator.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amila Kahvedžić et al.
Journal of medicinal chemistry, 56(2), 451-459 (2012-12-21)
In this paper we report the synthesis of a new family of hydroxyguanidinium aromatic derivatives (4a-g) as potential minor groove binders and cytotoxic agents. Their DNA affinity was evaluated by thermal denaturation experiments using salmon sperm DNA. The antiproliferative effects
Alycen Pond Nigro et al.
Archives of biochemistry and biophysics, 500(1), 66-73 (2010-03-30)
Yeast cytochrome c peroxidase was used to construct a model for the reactions catalyzed by the second cycle of nitric oxide synthase. The R48A/W191F mutant introduced a binding site for N-hydroxyguanidine near the distal heme face and removed the redox
Rémy Ricoux et al.
European journal of biochemistry, 270(1), 47-55 (2002-12-21)
Nitric oxide (NO) is a potent intra- and intercellular messenger involved in the control of vascular tone, neuronal signalling and host response to infection. In mammals, NO is synthesized by oxidation of l-arginine catalysed by hemeproteins called NO-synthases with intermediate
Zembowicz, A., et al.
British Journal of Pharmacology, 107, 1007-1007 (1992)
Kamil Brożewicz et al.
European journal of medicinal chemistry, 55, 384-394 (2012-08-16)
Twenty four 1-[2-alkylthio-5-(azol-2 or 5-yl)-4-chlorobenzenesulfonyl]-3-hydroxyguanidines 6a-x have been synthesized in order to evaluate their biological activity. Compounds 6a, 6c, 6d, 6f, 6g, 6i-p, 6r-t, and 6v-x were tested for their in vitro anticancer activity at the US National Cancer Institute. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service