227129
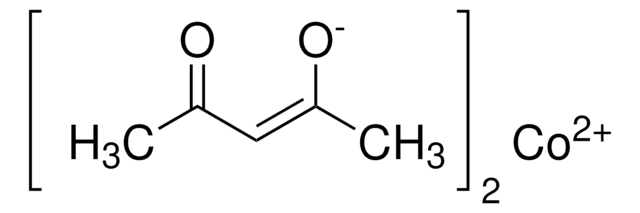
Cobalt(II) acetylacetonate
97%
Synonym(s):
2,4-Pentanedione cobalt(II) derivative, Bis(2,4-pentanedionato)cobalt, Co(acac)2, Cobaltous acetylacetonate
Select a Size
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder and chunks
reaction suitability
core: cobalt
impurities
≤3% water
mp
165-170 °C (lit.)
SMILES string
CC(=O)\C=C(\C)O[Co]O\C(C)=C/C(C)=O
InChI
1S/2C5H8O2.Co/c2*1-4(6)3-5(2)7;/h2*3,6H,1-2H3;/q;;+2/p-2/b2*4-3-;
InChI key
UTYYEGLZLFAFDI-FDGPNNRMSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | 932744 | 749230 | 688096 |
|---|---|---|---|
| form powder | form - | form solid | form powder |
| loss 0.5% loss on heating, 359°C (typical, TGA) | loss 0.5% TGA, >330°C (weight loss) | loss - | loss - |
| transition temp transition temp 437.6 °C (typical, DSC) | transition temp - | transition temp - | transition temp - |
| λmax 282 nm | λmax 287 nm±5 nm in dichloromethane, 373 nm±5 nm in dichloromethane | λmax - | λmax - |
| fluorescence λex 305 nm; λem 507 nm in chloroform | fluorescence λem 514 nm±10 nm in dichloromethane | fluorescence - | fluorescence λex 340 nm; λem 512 nm in chloroform |
General description
Application
- Cobalt (II)-Catalyzed Isocyanide Insertion Reaction with Amines: Details a synthetic method for forming ureas and azaheterocycles catalyzed by Cobalt(II) acetylacetonate, applicable in pharmaceutical synthesis (Zhu et al., 2014).
- Cobalt‐Catalyzed C−H Functionalizations by Imidate Assistance: Describes a method using Cobalt(II) acetylacetonate for C-H functionalization, important for organic synthesis and material chemistry (Mei & Ackermann, 2016).
- Cobalt (II) acetylacetonate covalently anchored onto magnetic mesoporous silica nanospheres: Focuses on its use as a catalyst for epoxidation of olefins, relevant for catalysis research (Li et al., 2015).
- A precursor in the solvothermal synthesis of Co3O4 nanoparticles. These nanoparticles exhibit high electrochemical performance and are used as a potential supercapacitor material due to their excellent capacitance and cycling stability.
- A precursor in the preparation of Co3O4 nanoparticles via hydrothermal method. The resulting Co3O4 nanoparticles exhibit a highly-uniform mesoporous structure and tunable sizes, making them promising for CO sensing applications.
- A precursor for the growth of cobalt oxide thin films using Metal-Organic Chemical Vapor Deposition (MOCVD).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Tris[2-phenylpyridinato-C2,N]iridium(III) sublimed grade](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)
![Tris[2-(p-tolyl)pyridine]iridium(III) ≥99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/957/bb807380-3690-46c2-809e-e6bc2ba29fa5/640/bb807380-3690-46c2-809e-e6bc2ba29fa5.png)
![Dichlorotetrakis[3,5-difluoro-2-(2-pyridinyl)phenyl]diiridium(III) 95%](/deepweb/assets/sigmaaldrich/product/structures/300/702/f03c66ed-f8fa-4103-a508-e57483592685/640/f03c66ed-f8fa-4103-a508-e57483592685.png)







