106399
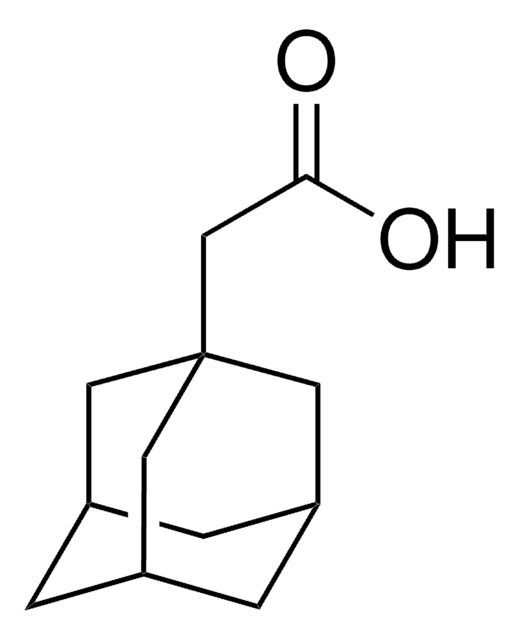
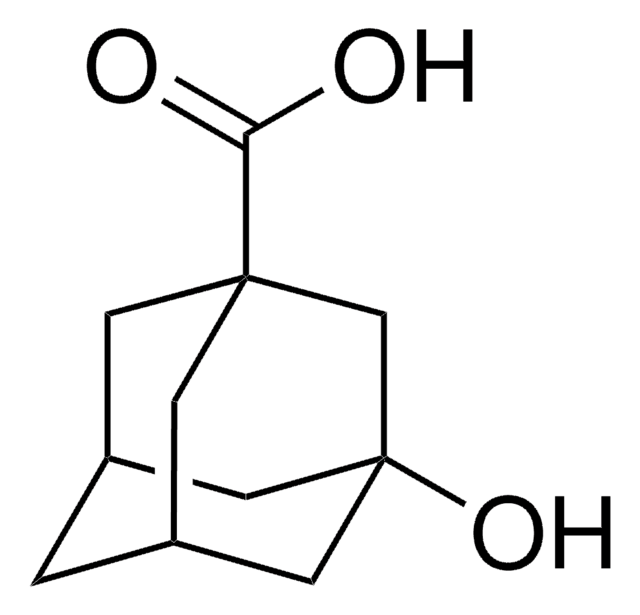
1-Adamantanecarboxylic acid
99%
Synonym(s):
Adamantane carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O2
CAS Number:
Molecular Weight:
180.24
Beilstein:
1910637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
172-174 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C11H16O2/c12-10(13)11-4-7-1-8(5-11)3-9(2-7)6-11/h7-9H,1-6H2,(H,12,13)/t7-,8+,9-,11-
InChI key
JIMXXGFJRDUSRO-KJZNFTALSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Adamantanecarboxylic acid can be used as:
- A stabilizer in the synthesis of monodisperse, highly crystalline CoPt3 nanoparticles and porous platinum nanoparticles.
- An additive in polycondensation reactions to yield conjugated polymers as possible optoelectronic materials.
- An additive in the allylic substitution reaction, which is catalyzed by palladium in an aqueous medium.
Biochem/physiol Actions
1-Adamantanecarboxylic acid undergoes complexation reactions with cyclohexaamylose. It is an inhibitor of phenyl ester hydrolysis of cycloheptaamylose.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Rozema et al.
Biochemistry, 35(49), 15760-15771 (1996-12-10)
Conditions that promote renaturation of an unfolded protein also promote protein aggregation, in many cases, because these competing intramolecular and intermolecular processes are driven by similar networks of noncovalent interactions. The GroEL/GroES system and related biological chaperones facilitate the renaturation
Nicolas Taulier et al.
The journal of physical chemistry. B, 112(31), 9546-9549 (2008-07-16)
We report temperature-dependent ultrasonic velocimetric and densimetric data on changes in volume, expansibility, and adiabatic compressibility associated with the binding of 1-adamantanecarboxylic acid (AD) to gamma-cyclodextrin (gamma-CD). We compare these results with our previous data on the binding of AD
Ying-Ming Zhang et al.
Bioorganic & medicinal chemistry, 18(4), 1415-1420 (2010-02-05)
A novel beta-cyclodextrin derivative 1 bearing 8-hydroxyquinolino and triazole groups was synthesized in satisfactory yield by 'click chemistry'. With a good water solubility up to 0.03 mol/L, 1 exhibited an effective switch-on fluorescence response to Cd(2+) over other common metal
Synthesis of porous platinum nanoparticles
Teng X, et al.
Small, 2(2), 249-253 (2006)
The complexation chemistry of cyclohexaamyloses: Adducts with 1-adamantanecarboxylic acid and anion.
Gelb RI, et al.
Bioorganic Chemistry, 9(4), 450-461 (1980)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service