11590
4,4′-Azobis(4-cyanovaleric acid)
≥98.0% (T)
Synonym(s):
4,4′-Azobis(4-cyanopentanoic acid), ABCVA, ACVA
About This Item
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
impurities
≤1% water
mp
118-125 °C (dec.) (lit.)
functional group
azo
carboxylic acid
nitrile
storage temp.
2-8°C
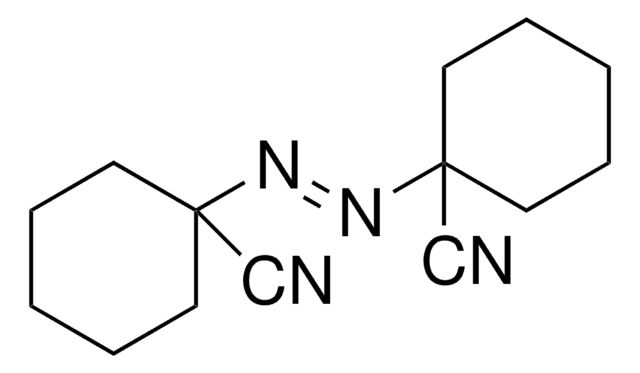
SMILES string
CC(CCC(O)=O)(\N=N\C(C)(CCC(O)=O)C#N)C#N
InChI
1S/C12H16N4O4/c1-11(7-13,5-3-9(17)18)15-16-12(2,8-14)6-4-10(19)20/h3-6H2,1-2H3,(H,17,18)(H,19,20)/b16-15+
InChI key
VFXXTYGQYWRHJP-FOCLMDBBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-react. D
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
An article regarding common FAQs for initiators and stabilizers.
Micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization.
Protocols
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)


![2-[[(2-Carboxyethyl)sulfanylthiocarbonyl]-sulfanyl]propanoic acid](/deepweb/assets/sigmaaldrich/product/structures/427/606/b02310e2-102e-4324-b09d-e4c0de4fab2c/640/b02310e2-102e-4324-b09d-e4c0de4fab2c.png)

