148938
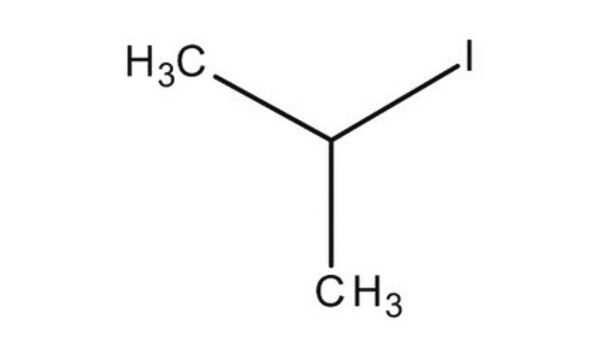
2-Iodopropane
contains copper as stabilizer, 99%
Synonym(s):
Isopropyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHI
CAS Number:
Molecular Weight:
169.99
Beilstein:
1098244
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.498 (lit.)
bp
88-90 °C (lit.)
mp
−90 °C (lit.)
density
1.703 g/mL at 25 °C (lit.)
functional group
alkyl halide
iodo
SMILES string
CC(C)I
InChI
1S/C3H7I/c1-3(2)4/h3H,1-2H3
InChI key
FMKOJHQHASLBPH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Iodopropane is an organ iodine compound. It is commonly used as a homolytic and heterolytic alkylating agent and in the preparation of the α-isopropoxymethylene blocking group. Additionally, it acts as an intermediator in the conversion of thiocarbonate to alkene.
Application
2-Iodopropane (Isopropyl iodide) was used to prepare butyric and isobutyric acid.
2-Iodopropane is used as an alkylating agent in the conversion of phenols, alcohols, oximes, carboxylic acids, and heteroatomic compounds (indoles, pyridines, purines, tetrazoles, pyrazoles, and amines) to give corresponding products.
2-Iodopropane is used as an alkylating agent in the conversion of phenols, alcohols, oximes, carboxylic acids, and heteroatomic compounds (indoles, pyridines, purines, tetrazoles, pyrazoles, and amines) to give corresponding products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Timur Coskun et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(21), 6840-6844 (2013-03-29)
Glycerol is converted to a mixture of butyric and isobutyric acid by rhodium- or iridium-catalysed carbonylation using HI as the co-catalyst. The initial reaction of glycerol with HI results in several intermediates that lead to isopropyl iodide, which upon carbonylation
Joe Rashan et al.
Journal of AOAC International, 86(4), 694-702 (2003-09-26)
An alternative liquid chromatographic (LC) method was developed and validated for the simultaneous determination of methoxyl and 2-hydroxypropoxyl substituents in hypromellose and hypromellose acetate succinate. The method uses the hydriodic acid cleavage reaction, catalyzed by adipic acid, of the substituted
2-Iodopropane
Wilson PD and Hilmey DG
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
W Kenealy et al.
Journal of bacteriology, 146(1), 133-140 (1981-04-01)
Iodopropane inhibited cell growth and methane production when Methanobacterium thermoautotrophicum, Methanobacterium formicicum, and Methanosarcina barkeri were cultured on H2-CO2. Iodopropane (40 microM) inhibited methanogenesis (30%) and growth (80%) when M. barkeri was cultured mixotrophically on H2-CO2-methanol. The addition of acetate
Development and validation of analytical procedure--control of residual 2-iodopropane in latanoprost.
Aleksandra Groman et al.
Acta poloniae pharmaceutica, 67(6), 673-676 (2011-01-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service