160695
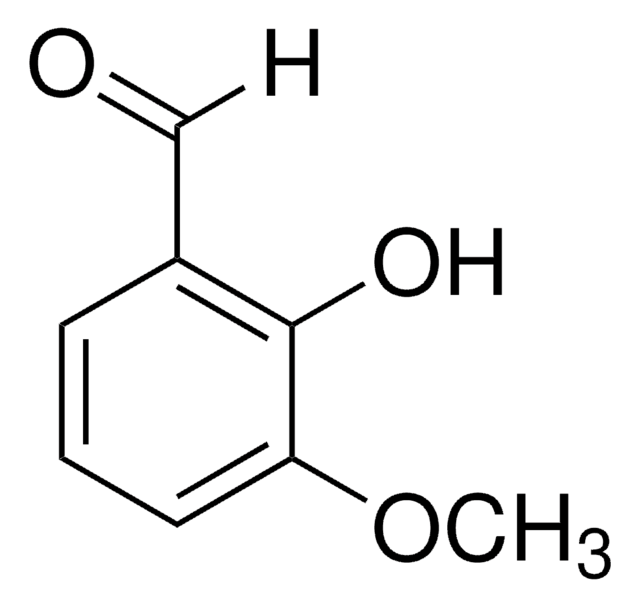
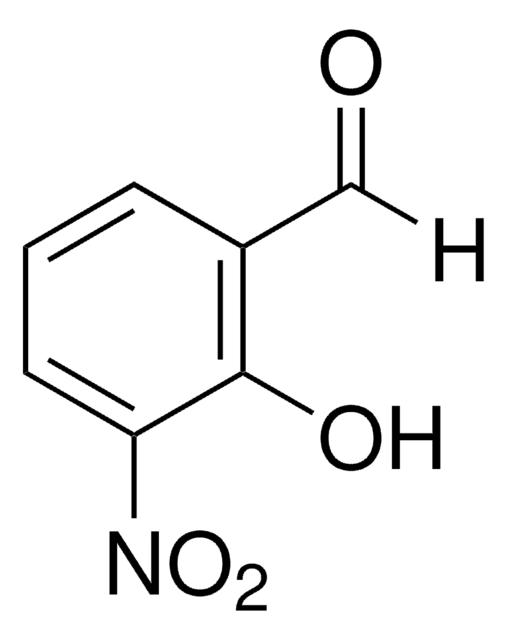
2-Hydroxy-4-methoxybenzaldehyde
98%
Synonym(s):
4-Methoxysalicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1072443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
41-43 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(OC)cc1O
InChI
1S/C8H8O3/c1-11-7-3-2-6(5-9)8(10)4-7/h2-5,10H,1H3
InChI key
WZUODJNEIXSNEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Hydroxy-4-methoxybenzaldehyde is the main component of root bark essential oil of Periploca sepium Bunge. It is a potential tyrosinase inhibitor present in African medicinal plants.
Application
2-Hydroxy-4-methoxybenzaldehyde was used in the synthesis of Schiff base ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Subban Nagarajan et al.
Journal of AOAC International, 86(3), 564-567 (2003-07-11)
The roots of Decalepis hamiltonii and Hemidesmus indicus are aromatic and possess the crystalline compound 2-hydroxy-4-methoxybenzaldehyde as the major compound (> 90%) in their volatile oils. A gas chromatographic procedure was developed for the assay of 2-hydroxy-4-methoxybenzaldehyde in both fresh
Belagihalli M Srikanta et al.
Biochimie, 93(4), 678-688 (2010-12-28)
Helicobacter pylori mediated gastric ulcer and cancers are common global problems since it was found to colonize in ∼50% of gastric ulcer/cancer patients. Decalepis hamiltonii, (Asclepiadaceae family) extracts have been depicted with medicinal properties supporting the traditional knowledge of health
P Giridhar et al.
Indian journal of experimental biology, 42(1), 106-110 (2004-07-28)
Axillary buds obtained from field grown plants of D. hamiltonii were used to initiate multiple shoots on Murashige and Skoog's medium (MS) supplemented with 2 mg L(-1) 6-benzyl aminopurine (BA) and 0.5 mg L(-1) indole-3-acetic acid (IAA). Profuse rooting was
I Kubo et al.
Planta medica, 65(1), 19-22 (1999-03-20)
By bioassay-guided fractionation using mushroom tyrosinase (EC 1.14.18.1), 2-hydroxy-4-methoxybenzaldehyde was characterized as the principal tyrosinase inhibitor from three East African medicinal plants, the root of Mondia whitei (Hook) Skeels (Asclepiaceae), the root of Rhus vulgaris Meikle (Anacardiaceae), and the bark
Gholamreza Karimipour et al.
Biological trace element research, 145(1), 109-117 (2011-08-13)
In this study, a new sorbent based on the gold nanoparticle loaded in activated carbon (Au-NP-AC) was synthesized and modified by bis(4-methoxy salicylaldehyde)-1,2-phenylenediamine (BMSAPD). This sorbent, which is abbreviated as Au-NP-AC-BMSAPD, has been applied for the enrichment and preconcentration of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service