All Photos(2)
About This Item
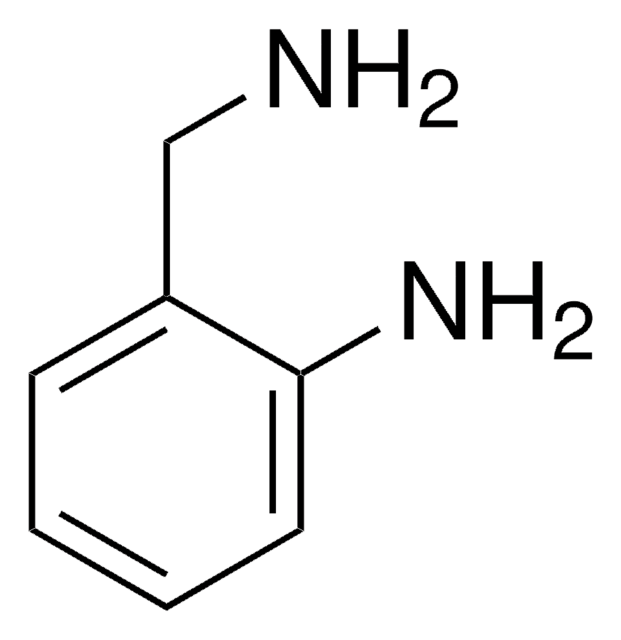
Linear Formula:
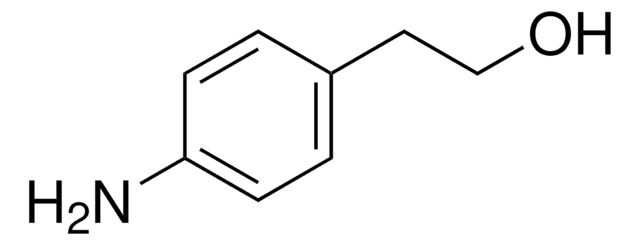
H2NC6H4CH2CH2OH
CAS Number:
Molecular Weight:
137.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.588 (lit.)
bp
147-148 °C/3.5 mmHg (lit.)
density
1.045 g/mL at 25 °C (lit.)
SMILES string
Nc1ccccc1CCO
InChI
1S/C8H11NO/c9-8-4-2-1-3-7(8)5-6-10/h1-4,10H,5-6,9H2
InChI key
ILDXSRFKXABMHH-UHFFFAOYSA-N
Related Categories
General description
2-Aminophenethyl alcohol undergoes one-pot cyclization with carboxylic acids in the presence of PPh3, CCl4 and NEt3 to yield N-acyl indolines.
Application
2-Aminophenethyl alcohol was used in the synthesis of:
- indole derivatives
- N-(cyanothioformyl)indoline
- dihydro-3,1-benzoxazepine
Other Notes
This material may darken over time with minimal impact to chemical purity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N-(Cyanothioformyl) indoline; a new indoline ring forming reaction.
Besson T, et al.
Journal of the Chemical Society. Perkin Transactions 1, 24, 4057-4060 (1998)
Zengxue Wang et al.
The Journal of organic chemistry, 72(24), 9364-9367 (2007-11-02)
A unique one-pot cyclization of 2-aminophenethyl alcohols with carboxylic acids in the presence of PPh3, CCl4, and NEt3 furnished the formation of N-acyl indolines in good to excellent yields. This new approach provides an efficient, scalable, low-cost, and direct access
Ruthenium-catalyzed dehydrogenative N-heterocyclization. Indoles from 2-aminophenethyl alcohols and 2-nitrophenethyl alcohols.
Tsuji Y, et al.
The Journal of Organic Chemistry, 55(2), 580-584 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service