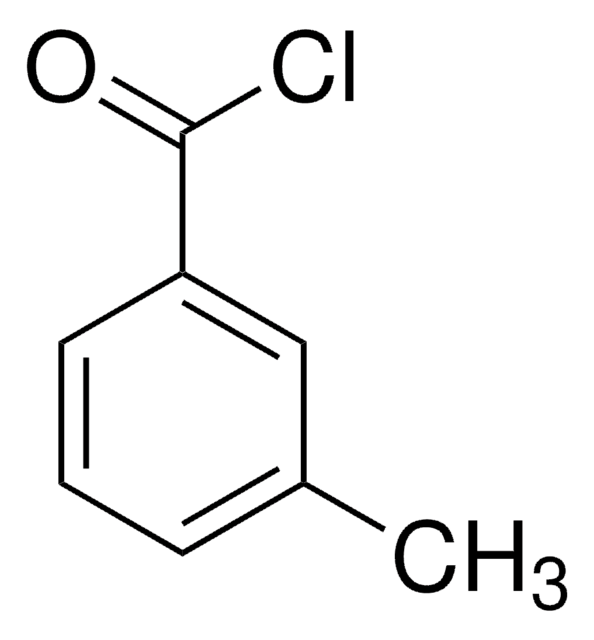
328014
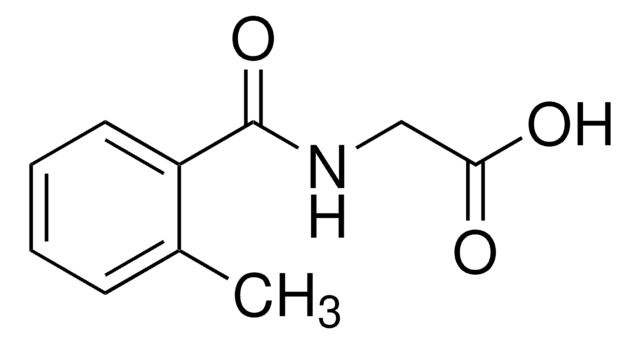
3-Methylhippuric acid
98%
Synonym(s):
N-(3-Methylbenzoyl)glycine, m-Toluric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4CONHCH2CO2H
CAS Number:
Molecular Weight:
193.20
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
138-140 °C (lit.)
SMILES string
Cc1cccc(c1)C(=O)NCC(O)=O
InChI
1S/C10H11NO3/c1-7-3-2-4-8(5-7)10(14)11-6-9(12)13/h2-5H,6H2,1H3,(H,11,14)(H,12,13)
InChI key
YKAKNMHEIJUKEX-UHFFFAOYSA-N
Related Categories
General description
3-Methylhippuric acid is also referred as m-methyl-hippuric acid. It is major product of xylene biotransformation in urine.
Application
3-Methylhippuric acid was employed as biological marker in studies on occupational exposure to xylene (solvent).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D de Carvalho et al.
International archives of occupational and environmental health, 63(1), 33-37 (1991-01-01)
The industrial solvents, toluene and xylene, have physicochemical properties that can be hazardous to the workers exposed. Since hippuric acid and m-methyl-hippuric acid represent the products of toluene and xylene biotransformation in urine, they are used as biological markers in
R Tardif et al.
Occupational and environmental medicine, 51(3), 187-191 (1994-03-01)
This study was undertaken to determine whether previous subacute treatment with ethanol could modify the kinetics of m-xylene in humans. A group of six volunteers was exposed twice to either 100 or 400 ppm of m-xylene during two hours (between
Determination of methylhippuric acid in human urine by high-performance liquid chromatography and by isotachophoresis.
J Sollenberg et al.
Journal of chromatography, 343(2), 419-423 (1985-10-11)
Possible preferential metabolism of xylene isomers following occupational exposure to mixed xylenes.
M J Miller et al.
International archives of occupational and environmental health, 72(2), 89-97 (1999-04-10)
Solvent exposures commonly involve mixtures of substances or mixtures of isomers of a single solvent. These may be metabolised through common pathways, resulting in the potential for metabolic interactions. These may then lead to accumulation of solvent or metabolic intermediates
A Astier
Journal of chromatography, 573(2), 318-322 (1992-01-17)
A high-performance liquid chromatographic method is described for the simultaneous determination of six urinary metabolites of several aromatic chemicals: phenol (from benzene), hippuric acid (from toluene), 3-methylhippuric acid (from xylene), mandelic and phenylglyoxylic acid (from styrene) and 4-nitrophenol (from nitrobenzene).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service