All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4N4
CAS Number:
Molecular Weight:
120.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
208 °C (dec.) (lit.)
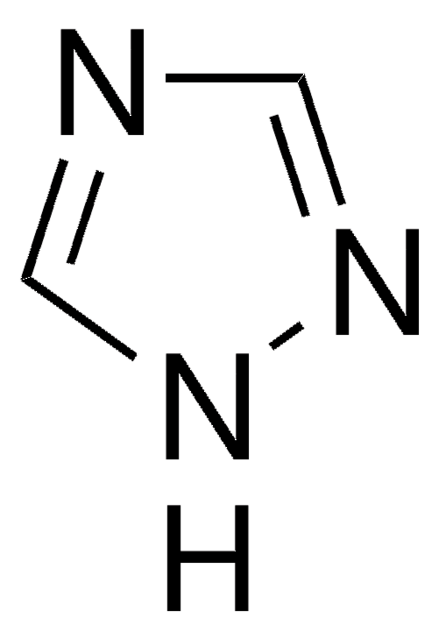
SMILES string
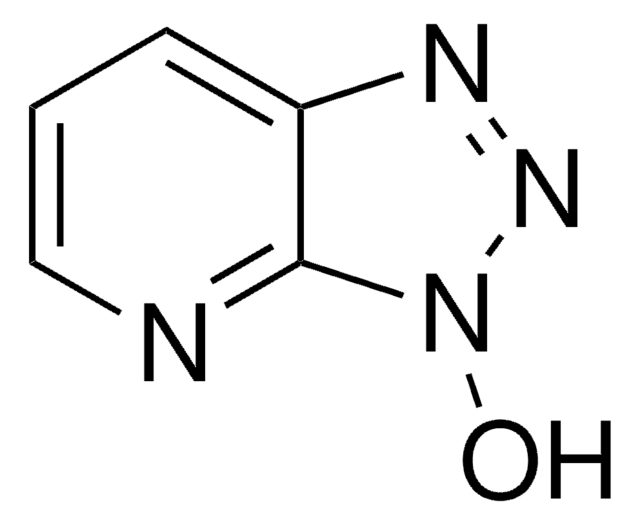
c1cnc2nn[nH]c2c1
InChI
1S/C5H4N4/c1-2-4-5(6-3-1)8-9-7-4/h1-3H,(H,6,7,8,9)
InChI key
VQNDBXJTIJKJPV-UHFFFAOYSA-N
General description
1H-1,2,3-Triazolo[4,5-b]pyridine belongs to the class of triazolopyridine. It reacts with europium under solvothermal conditions in pyridine to yield the homoleptic framework containing EuII centers that are icosahedrally coordinated by the 12 nitrogen atoms of six chelating ligands. The surface-enhanced Raman (SER) spectra of 1H-1,2,3-triazolo[4,5-b]pyridine adsorbed on silver hydrosols is studied in the region 3500-100cm-1.
Application
1H-1,2,3-Triazolo[4,5-b]pyridine (1,2,3-Triazolo(5,4-b)pyridine) may be used:
- as starting reagent in the synthesis of 2,4,8,10-tetranitro-5H-pyrido[3″,2″:4′,5′] [1,2,3] triazolo [1′,2′:1,2] [1,2,3]- triazolo [5,4-b]-pyridin-6-ium inner salt
- in the pesticide synthesis
- as an entry to mesoionic heteropentalene derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
(Infinity)3[Eu(Tzpy)2]: a homoleptic framework containing [Eu(II)N12] icosahedra.
Klaus Müller-Buschbaum et al.
Angewandte Chemie (International ed. in English), 46(23), 4385-4387 (2007-05-05)
The Chemistry of the [1, 2, 3] Triazolo [1, 5-a] pyridines: An Update.
Jones G and Abarca B.
Advances in Heterocyclic Chemistry, 100, 195-252 (2010)
Yifang Zhang et al.
Talanta, 219, 121356-121356 (2020-09-06)
Chemical derivatization of glycans is a common strategy to increase the analytical performance of MALDI-MS-based glycan profiling techniques. Hydrazide, one of the most popular tags, offers important advantages including allowing purification-free procedures. Several hydrazides have thus been used for glycomics
Bull. Soc. Chim. Belg., 95, 1107-1107 (1986)
Helvetica Chimica Acta, 58, 1521-1521 (1975)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service