431982
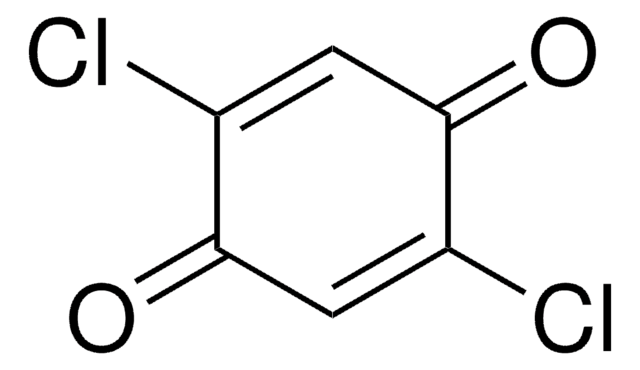
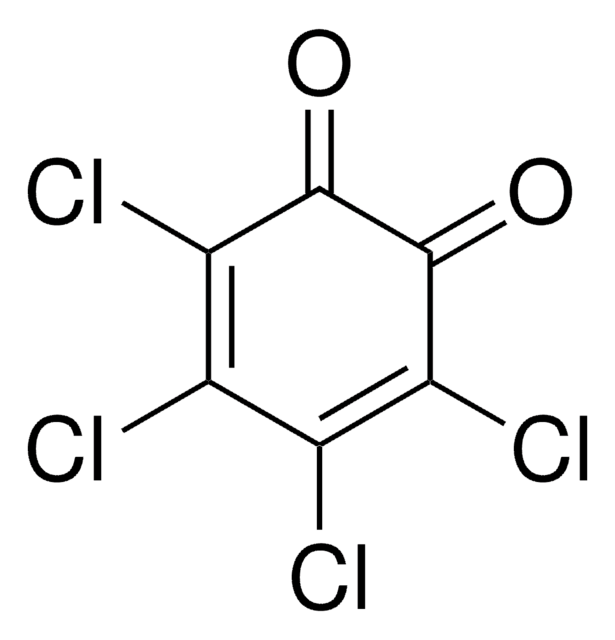
2,6-Dichloro-1,4-benzoquinone
98%
Synonym(s):
2,6-Dichloro-2,5-cyclohexadiene-1,4-dione, 2,6-Dichloro-p-benzoquinone, 2,6-Dichlorobenzoquinone, 2,6-Dichloroquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H2Cl2O2
CAS Number:
Molecular Weight:
176.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
122-124 °C (lit.)
SMILES string
ClC1=CC(=O)C=C(Cl)C1=O
InChI
1S/C6H2Cl2O2/c7-4-1-3(9)2-5(8)6(4)10/h1-2H
InChI key
JCARTGJGWCGSSU-UHFFFAOYSA-N
Related Categories
General description
2,6-Dichloro-1,4-benzoquinone (DCBQ, 2,6-DCBQ) is a halobenzoquinone. Halobenzoquinones are disinfection byproducts (DBPs) found in drinking water. They are capable of producing reactive oxygen species and causing oxidative damage to proteins and DNA in T24 human bladder carcinoma cells. UV-induced transformation of DCBQ in drinking water has been reported. Detection of DCBQ in drinking water by liquid chromatography-ESI tandem mass spectrometry has been reported.
Application
2,6-Dichloro-1,4-benzoquinone is the suitable reagent used to in a study to evaluate the diffusion coefficients (D) for a family of quinones, nitroaromatics, ferrocenes and aromatic hydrocarbon compounds, in acetonitrile by single potential step chronoamperometry. It may be used in the preparation of :
- 2,3,5-Trichloro-1,4-dihydroquinone
- 2,3,5-trichloro-1,4-benzoquinone
- 2,6-Dichloro-1,4-dihydroquinone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A A Leontievsky et al.
Biodegradation, 11(5), 331-340 (2001-08-07)
The toxicity of thirteen isomers of mono-, di-, tri- and pentachlorophenols was tested in potato-dextrose agar cultures of the white rot fungi Panus tigrinus and Coriolus versicolor. 2,4,6-Trichlorophenol (2,4,6-TCP) was chosen for further study of its toxicity and transformation in
N Knoepfle et al.
Biochemistry, 38(5), 1582-1588 (1999-02-04)
The intrinsic chlorophyll protein CP 43, a component of photosystem II (PS II) in higher plants, green algae, and cyanobacteria, is encoded by the psbC gene. Oligonucleotide-directed mutagenesis was employed to introduce mutations into a segment of psbC that encodes
Yichao Qian et al.
Environmental science & technology, 47(9), 4426-4433 (2013-04-09)
Halobenzoquinones (HBQs) are a group of emerging disinfection byproducts (DBPs) found in treated drinking water. Because the use of UV treatment for disinfection is becoming more widespread, it is important to understand how the HBQs may be removed or changed
Ben-Zhan Zhu et al.
Proceedings of the National Academy of Sciences of the United States of America, 104(10), 3698-3702 (2007-03-16)
The metal-independent decomposition of organic hydroperoxides and the formation of organic alkoxyl radicals in the absence or presence of halogenated quinones were studied with electron spin resonance (ESR) and the spin-trapping agent 5,5-dimethyl-1-pyrroline N-oxide (DMPO). We found that 2,5-dichloro-1,4-benzoquinone (DCBQ)
Yasuhiro Kashino et al.
Biochemistry, 41(25), 8004-8012 (2002-06-19)
A highly active oxygen-evolving photosystem II (PSII) complex was purified from the HT-3 strain of the widely used cyanobacterium Synechocystis sp. PCC 6803, in which the CP47 polypeptide has been genetically engineered to contain a polyhistidine tag at its carboxyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service