472654
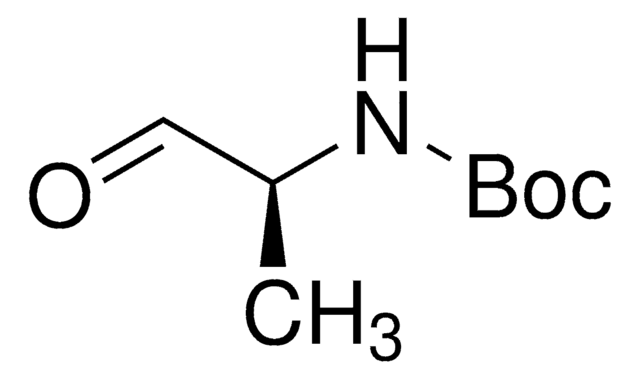
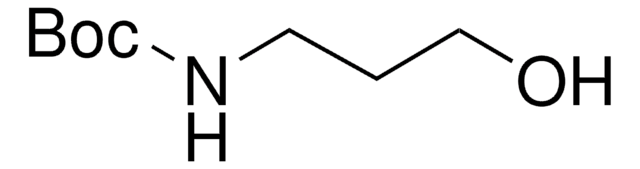
N-Boc-2-aminoacetaldehyde
95%
Synonym(s):
tert-Butyl N-(2-oxoethyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HCOCH2NHCO2C(CH3)3
CAS Number:
Molecular Weight:
159.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.455 (lit.)
storage temp.
−20°C
SMILES string
CC(C)(C)OC(=O)NCC=O
InChI
1S/C7H13NO3/c1-7(2,3)11-6(10)8-4-5-9/h5H,4H2,1-3H3,(H,8,10)
InChI key
ACNRTYKOPZDRCO-UHFFFAOYSA-N
Gene Information
human ... CTSK(1513)
General description
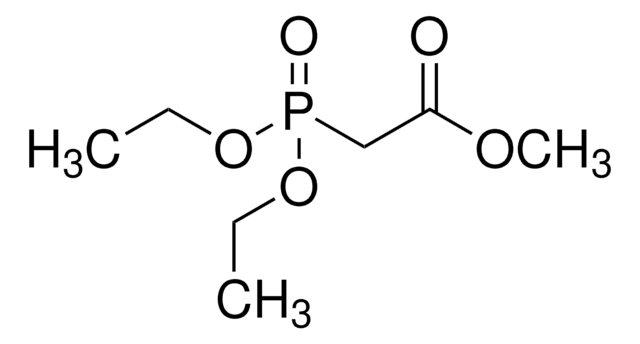
N-Boc-2-aminoacetaldehyde is an organic building block. It reacts with Horner-Wadsworth-Emmons (HWE) reagent to afford γ-aminobutyric acid (GABA)-derived α-keto amide/ester units.
Application
α-Methylenation of this amino aldehyde proceeds in a quick and efficient manner using a recently reported protocol involving formaldehyde and catalysis by either pyrrolidine proprionic acid or the dipeptide L-Pro-β-Ala.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
Also used in a three-component synthesis of pyrrolidines involving 1,3-dipolar cycloaddition.
N-Boc-2-aminoacetaldehyde may be employed in the following:
- As a starting reagent in the total synthesis of (+)-negamycin.
- Synthesis of (E)-ethyl 4-((tert-butoxycarbonyl)amino)but-2-enoate.
- Synthesis of 2,2′-bipyridine.
A building block in the synthesis of a protected pyrroloproline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Efficient total synthesis of (+)-negamycin, a potential chemotherapeutic agent for genetic diseases.
Yoshio Hayashi et al.
Chemical communications (Cambridge, England), (20)(20), 2379-2381 (2008-05-14)
Herein, we describe an efficient strategy for the total synthesis of (+)-negamycin using commercially available achiral N-Boc-2-aminoacetaldehyde as starting material with 42% overall yield for a limited number of steps.
Diethyl [3-Cyano-2-Oxo-3-(Triphenylphosphoranylidene) propyl] phosphonate: A Useful Horner-Wadsworth-Emmons Reagent for alpha-Keto (Cyanomethylene)-triphenylphosphoranes from Carbonyl Compounds.
Lee K.
Bull. Korean Chem. Soc., 28(10), 1641-1641 (2007)
Anniina Erkkilä et al.
The Journal of organic chemistry, 71(6), 2538-2541 (2006-03-11)
A rapid and extremely convenient method for alpha-methylenation of aldehydes with aqueous formaldehyde is described. Two optimal catalytic systems are presented that allow short reaction times and afford the functionalized products in good to excellent yields (up to 99%) and
Anna Turetsky et al.
Scientific reports, 4, 4782-4782 (2014-04-25)
A number of Bruton's tyrosine kinase (BTK) inhibitors are currently in development, yet it has been difficult to visualize BTK expression and pharmacological inhibition in vivo in real time. We synthesized a fluorescent, irreversible BTK binder based on the drug
Claudia Karnthaler-Benbakka et al.
Angewandte Chemie (International ed. in English), 53(47), 12930-12935 (2014-08-01)
The development of receptor tyrosine-kinase inhibitors (TKIs) was a major step forward in cancer treatment. However, the therapy with TKIs is limited by strong side effects and drug resistance. The aim of this study was the design of novel epidermal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service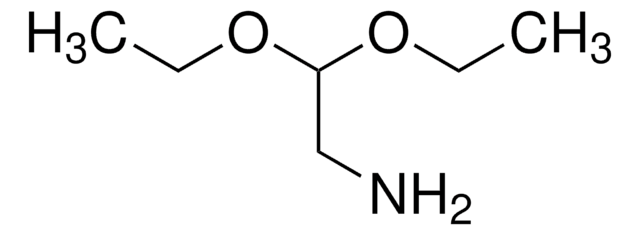
![3-[(Benzyloxycarbonyl)amino]propionaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/408/203/100fb0f0-7072-41be-b6e0-2857cdc324ee/640/100fb0f0-7072-41be-b6e0-2857cdc324ee.png)