663190
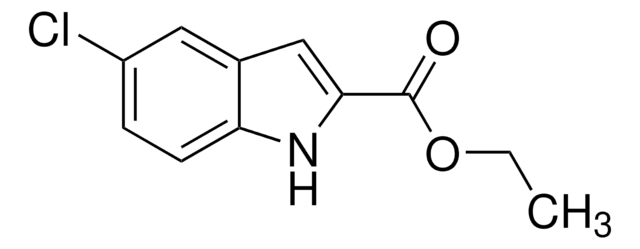
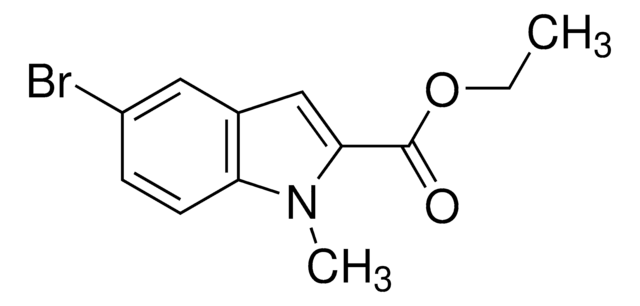
Ethyl 5-methylindole-2-carboxylate
97%
Synonym(s):
5-Methylindole-2-carboxylic acid ethyl ester, NSC 30928
About This Item
Recommended Products
Assay
97%
form
solid
mp
160-164 °C
SMILES string
CCOC(=O)c1cc2cc(C)ccc2[nH]1
InChI
1S/C12H13NO2/c1-3-15-12(14)11-7-9-6-8(2)4-5-10(9)13-11/h4-7,13H,3H2,1-2H3
InChI key
KMVFKXFOPNKHEM-UHFFFAOYSA-N
General description
Application
- Reactant for synthesis of oxazino[4,3-a]indoles via cascade addition-cyclization reactions
- Reactant for preparation of indolecarboxamides as cannabinoid CB1 receptor antagonists
- Reactant for preparation of indole-3-propionic acids as antiinflammatory and analgesic agents
- Reactant for Friedel-Crafts acylation with nitrobenzoyl chloride
- Reactant for oximation reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service