796557
Stahl Aerobic Oxidation ABNO solution
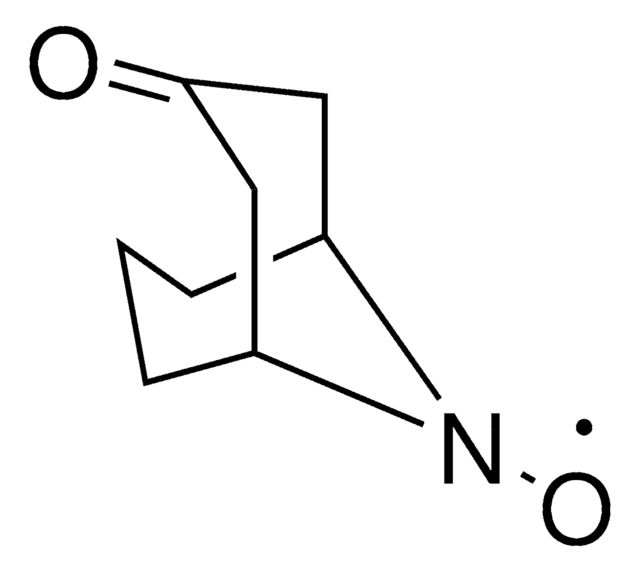
0.04M ABNO in Acetonitrile, Solution for Oxidation of Primary and Secondary Alcohols
About This Item
Recommended Products
description
Freezing Point: 44.6°F
form
liquid
reaction suitability
reagent type: oxidant
refractive index
n/D 1.357
density
0.796 at 25 °C
storage temp.
2-8°C
Related Categories
General description
Application
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
43.5 °F
Flash Point(C)
6.38 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Related Content
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)
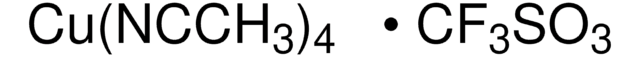


![1-((3,5-Difluorophenyl)sulfonyl)bicyclo[1.1.0]butane 95%](/deepweb/assets/sigmaaldrich/product/structures/640/054/e4a2fe87-4239-49ea-be2f-1273316c36ba/640/e4a2fe87-4239-49ea-be2f-1273316c36ba.png)








