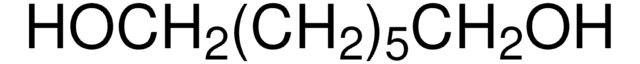H11807
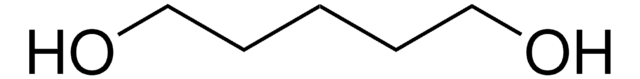
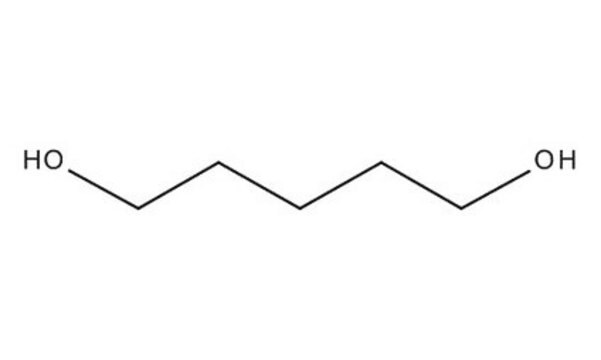
1,6-Hexanediol
97%
Synonym(s):
Hexamethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
HO(CH2)6OH
CAS Number:
Molecular Weight:
118.17
Beilstein:
1633461
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.53 mmHg ( 20 °C)
Quality Level
Assay
97%
autoignition temp.
608 °F
expl. lim.
16 %
bp
250 °C (lit.)
mp
38-42 °C (lit.)
SMILES string
OCCCCCCO
InChI
1S/C6H14O2/c7-5-3-1-2-4-6-8/h7-8H,1-6H2
InChI key
XXMIOPMDWAUFGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,6-Hexanediol is generally used to introduce C6-spacer in molecular substrates. It is also widely used in the synthesis of various polyesters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Melt-phase synthesis and properties of triptycene-containing copolyesters.
Liu, Yanchun et al.
Macromolecules, 44(11), 4049-4056 (2011)
Clinton Belott et al.
Proceedings of the National Academy of Sciences of the United States of America, 117(44), 27676-27684 (2020-10-21)
Proteinaceous liquid-liquid phase separation (LLPS) occurs when a polypeptide coalesces into a dense phase to form a liquid droplet (i.e., condensate) in aqueous solution. In vivo, functional protein-based condensates are often referred to as membraneless organelles (MLOs), which have roles
Kifah Nasr et al.
Polymers, 12(9) (2020-08-28)
Among the various catalysts that can be used for polycondensation reactions, enzymes have been gaining interest for three decades, offering a green and eco-friendly platform towards the sustainable design of renewable polyesters. However, limitations imposed by their delicate nature, render
A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources.
Jiang, Min et al.
Journal of Polymer Science Part A: Polymer Chemistry, 50(5), 1026-1036 (2012)
Liquid crystalline phthalocyanine-fullerene dyads.
Ince, Mine et al.
Journal of Materials Chemistry, 21(5), 1531-1536 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service