W391018
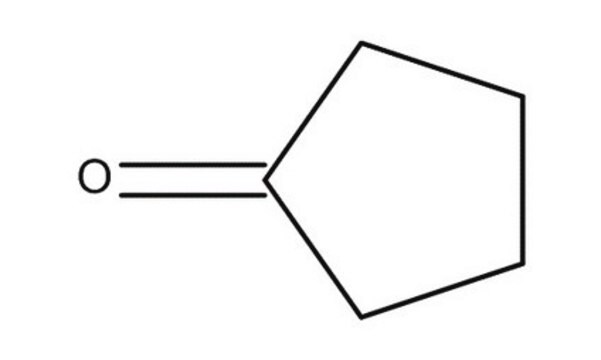
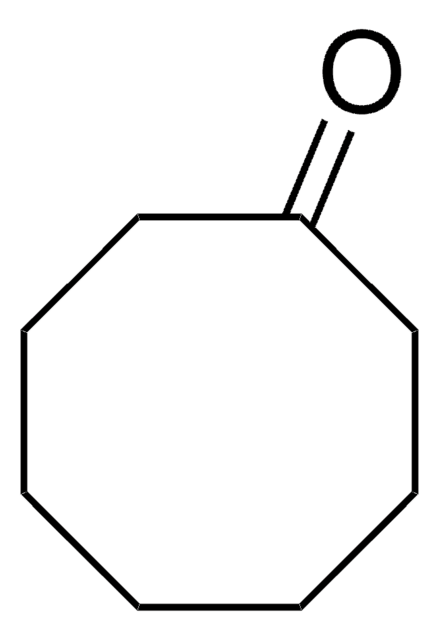
Cyclopentanone
≥99%, FG
Synonym(s):
Adipic ketone, Dumasin, Ketopentamethylene
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 110
Assay
≥99%
refractive index
n20/D 1.437 (lit.)
bp
130-131 °C (lit.)
mp
−51 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
minty
SMILES string
O=C1CCCC1
InChI
1S/C5H8O/c6-5-3-1-2-4-5/h1-4H2
InChI key
BGTOWKSIORTVQH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- High-oxygen-modified atmospheric packaging delays flavor and quality deterioration in fresh-cut broccoli.: This research investigates the impact of high-oxygen-modified atmospheric packaging on the preservation of fresh-cut broccoli. Cyclopentanone is identified as a significant compound in the aroma profile, contributing to the understanding of packaging technologies that maintain food quality and extend shelf life. (He et al., 2024).
- Selective production of methylindan and tetralin with xylose or hemicellulose.: The study focuses on the selective production of methylindan and tetralin using xylose or hemicellulose, with Cyclopentanone playing a key role in the catalytic processes. This research provides insights into sustainable chemical production from biomass-derived feedstocks. (Zou et al., 2024).
- Synthesis of Hydroxylamine via Ketone-Mediated Nitrate Electroreduction.: This paper presents a novel method for synthesizing hydroxylamine through ketone-mediated nitrate electroreduction, utilizing Cyclopentanone. The findings demonstrate the versatility of Cyclopentanone in electrochemical synthesis and its potential for green chemistry applications. (Jia et al., 2024).
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
86.0 °F - closed cup
Flash Point(C)
30 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service