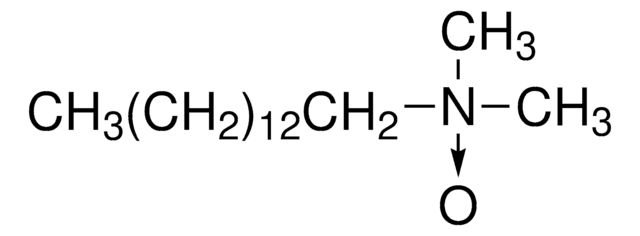108693
Deviron® C16
Detergent 30% solution, EMPROVE® EVOLVE
Pharma Manufacturing
Synonym(s):
Deviron® C16 Detergent EMPROVE® EVOLVE solution, N,N-Dimethylmyristylamine N-oxide
About This Item
Recommended Products
Quality Level
product line
EMPROVE® EVOLVE
form
liquid
greener alternative product characteristics
Designing Safer Chemicals
Use of Renewable Feedstocks
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
application(s)
pharma/biopharma processes
greener alternative category
storage temp.
15-25°C
InChI
1S/C16H35NO/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17(2,3)18/h4-16H2,1-3H3
InChI key
ONHFWHCMZAJCFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Our SAFC portfolio of high-quality raw materials for use in biopharmaceutical processing withstands strict quality control procedures plus the documentation and expertise to help our customers meet requirements as defined by the M-Clarity Program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
We offer a range of high quality critical raw materials plus the documentation and expertise to help our customers meet stringent regulatory requirements. Our SAFC portfolio of high-quality products withstands strict quality control procedures.
Application
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
212.0 °F
Flash Point(C)
> 100 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This page describes key considerations for cell lysis and how the combination of a high salt concentration and a salt tolerant endonuclease can be used to increase vector titer and infectivity during AAV vector manufacturing.
Related Content
The use of Benzonase® endonuclease can significantly reduce the levels of DNA by more than 100,000-fold while also reducing viscosity and protecting downstream equipment from DNA fouling. However, optimization strategies and DoE are critical when it comes to reducing DNA in your process. Setting up a DoE for your Benzonase® endonuclease application can help you find the optimal operation conditions that deliver the required DNA clearance from your process.
Learn more on mAb downstream processing, more specifically virus inactivation and the relevant associated products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service