All Photos(1)
About This Item
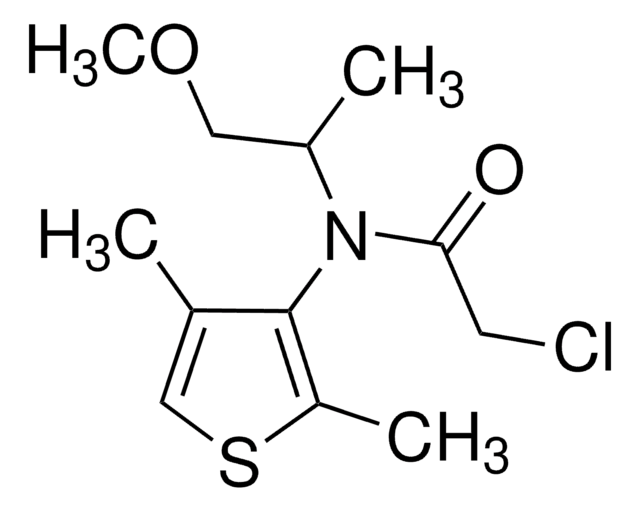
Empirical Formula (Hill Notation):
C11H19N3O
CAS Number:
Molecular Weight:
209.29
Beilstein:
882476
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
CCCCc1c(C)nc(NCC)nc1O
InChI
1S/C11H19N3O/c1-4-6-7-9-8(3)13-11(12-5-2)14-10(9)15/h4-7H2,1-3H3,(H2,12,13,14,15)
InChI key
BBXXLROWFHWFQY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Chromatographic determination of Milgo in water, soil, and plants].
A A Krasnykh et al.
Gigiena i sanitariia, (6)(6), 69-70 (1980-06-01)
P Proença et al.
Forensic science international, 133(1-2), 95-100 (2003-05-14)
Fenarimol (Rubigan) is a pyrimidine ergosterol biosynthesis inhibitor used as a systemic fungicide. The authors present a fatal fenarimol intoxication case analysed in the Forensic Toxicology Service of the National Institute of Legal Medicine. The results were used to compare
L J Fitzpatrick et al.
Journal of chromatography. A, 918(2), 429-433 (2001-06-16)
This paper assesses the effect of pressurised fluid extraction (PFE) on the recovery of bupirimate and its degradation product, ethirimol from a range of soil types. The analytes were extracted under standard conditions (pressure, 2000 p.s.i.; temperature, 100 degrees C;
Alexander I Vardavas et al.
Mutation research, 829-830, 26-32 (2018-05-01)
Imidacloprid (IMI) is a systemic, chloro-nicotinyl insecticide classified in Regulation N° 1272/2008 of the European Commision as "harmful if swallowed and very toxic to aquatic life, with long-lasting effects". IMI is metabolized in vitro both by aldehyde oxidase (AOX) (reduction)
Shagufta Khan et al.
Food chemistry, 199, 870-875 (2016-01-19)
In the present communication, a non-covalent fenarimol-imprinted polymer was synthesized by precipitation polymerization technique using methacrylic acid (MAA) as a functional monomer, ethylene glycol dimethacrylate (EGDMA) as a cross-linker, and azobisisobutyronitrile (AIBN) as an initiator in different porogenic solvent. Binding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service