86040
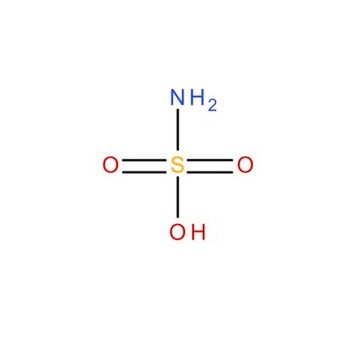
Sulfamic acid
analytical standard (for acidimetry), ACS reagent
Synonym(s):
Aminosulfonic acid, Sulphamic acid, Amidosulfonic acid
About This Item
Recommended Products
grade
ACS reagent
analytical standard (for acidimetry)
Quality Level
Agency
complies with DIN 19266
Assay
99.3-100.3% (dried material)
mp
215-225 °C (dec.) (lit.)
anion traces
chloride (Cl-): ≤10 mg/kg
sulfate (SO42-): ≤500 mg/kg
cation traces
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤10 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
application(s)
environmental
food and beverages
general analytical
industrial qc
pharmaceutical
format
mixture
SMILES string
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
InChI key
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Sulfamic acid also finds its use as a primary standard in non-aqueous visual, conductometric, and potentiometric titrations.
Features and Benefits
- Available in a secure glass bottle to ensure its stability for the entire shelf life until opened.
- High-quality offering accurate titer determinations
- Accompanied by a detailed certificate of analysis (CoA)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service