Y0000468
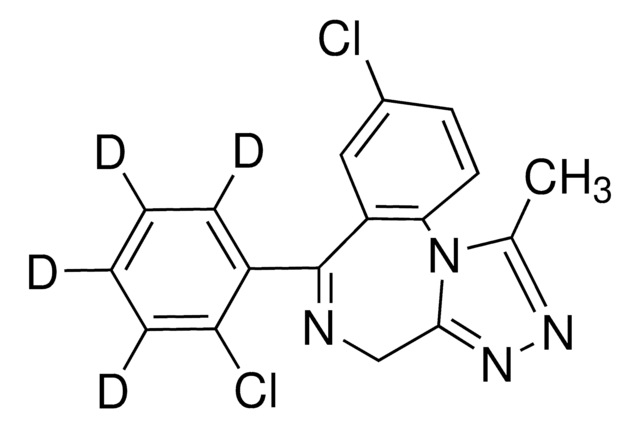
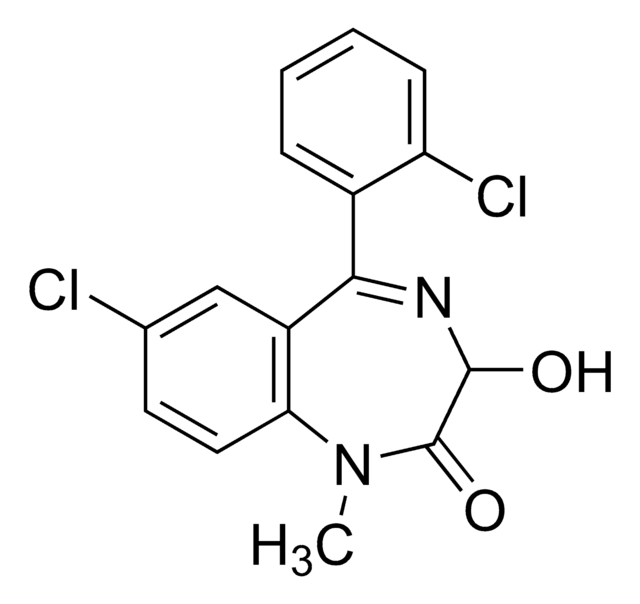
Brotizolam
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H10BrClN4S
CAS Number:
Molecular Weight:
393.69
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
brotizolam
manufacturer/tradename
EDQM
drug control
psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C15H10BrClN4S/c1-8-19-20-13-7-18-14(9-4-2-3-5-11(9)17)10-6-12(16)22-15(10)21(8)13/h2-6H,7H2,1H3
InChI key
UMSGKTJDUHERQW-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Brotizolam EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Tanaka et al.
Methods and findings in experimental and clinical pharmacology, 30(8), 607-613 (2008-12-18)
In this study we examined the behavioral pharmacological side effects after recovery from the loss of righting reflex induced by three benzodiazepine receptor agonists - zolpidem, brotizolam and flunitrazepam - in ddY mice. All agents caused marked motor incoordination in
Hiromi Katoh et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 127(12), 2035-2044 (2007-12-07)
In the present study, we tested three kinds of sleeping drugs, consisting mainly of triazolam, brotizolam, and flunitrazepam, to compare the drug efficacy of generic drugs with that of original drugs. After these drugs were administered orally to mice, drug
C G Van Reenen et al.
Physiology & behavior, 96(2), 307-314 (2008-11-11)
The present study examined the effects of the intravenous administration of the anxiolytic drug brotizolam on the behavioral and physiological responsiveness of calves to novelty in a dose response fashion. Holstein Friesian heifer calves (39-41 weeks of age; body weight
Naoko Tanaka et al.
Soudni lekarstvi, 56(1), 5-6 (2011-03-19)
A case of drowning involving brotizolam, flunitrazepam and ethanol ingestion was presented. Quantitative toxicological analysis showed that the concentrations of brotizolam, 7-aminoflunitrazepam (a metabolite of flunitrazepam) and ethanol in the femoral blood were 0.025 microg/ml, 0.094 microg/ml and 0.29 mg/ml
Atsushi Takiguchi et al.
Journal of pharmacological sciences, 101(4), 325-328 (2006-08-08)
Triazolam caused no significant increase in the total error at 0.05 and 0.1 mg/kg. However, at 0.2 mg/kg, it caused a significant increase in total error. Almost the same findings were observed with brotizolam and rilmazafone. That is, at 0.2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service